Multiple sclerosis, a treatable disease
- PMID: 29196354
- PMCID: PMC6297710
- DOI: 10.7861/clinmedicine.17-6-530
Multiple sclerosis, a treatable disease
Abstract
This article reviews our current understanding and modern treatment of multiple sclerosis (MS). MS is a disabling condition resulting in devastating social and economic impacts. As MS can affect any part of the central nervous system, the presentation is often diverse; however, there are key features that can be useful in the clinic. We comment on the diagnostic criteria and review the main subtypes of MS, including clinically isolated syndrome, relapsing remitting MS, secondary progressive MS and primary progressive MS. Although the underlying aetiology of MS is still not known, we summarise those with most evidence of association. Finally, we aim to present treatment strategies for managing the acute relapse, disease-modifying therapies and MS symptoms. This review highlights that progressive MS is an area where there is currently a paucity of available disease-modifying treatments and this will be a major focus for future development.
Keywords: Diagnostic criteria; epidemiology; multiple sclerosis; progressive multiple sclerosis; treatments.
© Royal College of Physicians 2017. All rights reserved.
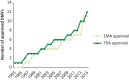
Figures

References
-
- Multiple Sclerosis International Federation Atlas of MS mapping multiple sclerosis around the world. London: MSIF, 2013. 2013
-
- Compston A. Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. - PubMed
-
- Stevenson EV. Alexander JS. Yun JW. Becker F. Gonzalez-Toledo E. Chapter 16 – Mechanisms of blood–brain barrier disintegration in the pathophysiology of multiple sclerosis. In: Minagar A, editor. Multiple sclerosis. London:: Academic Press; 2016. pp. 393–413.
-
- Deangelis TM. Miller A. Diagnosis of multiple sclerosis. In: Tselis AC, editor; Booss J, editor. Handbook of clinical neurology. Amsterdam:: Elsevier BV; 2014. pp. 307–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

