Sleep Treatment Outcome Predictors (STOP) Pilot Study: a protocol for a randomised controlled trial examining predictors of change of insomnia symptoms and associated traits following cognitive-behavioural therapy for insomnia in an unselected sample
- PMID: 29196479
- PMCID: PMC5719290
- DOI: 10.1136/bmjopen-2017-017177
Sleep Treatment Outcome Predictors (STOP) Pilot Study: a protocol for a randomised controlled trial examining predictors of change of insomnia symptoms and associated traits following cognitive-behavioural therapy for insomnia in an unselected sample
Abstract
Introduction: Cognitive-behavioural therapy for insomnia (CBT-I) leads to insomnia symptom improvements in a substantial proportion of patients. However, not everyone responds well to this treatment, and it is unclear what determines individual differences in response. The broader aim of this work is to examine to what extent response to CBT-I is due to genetic and environmental factors. The purpose of this pilot study is to examine feasibility of a design to test hypotheses focusing on an unselected sample, that is, without selection on insomnia complaints, in order to plan a larger behavioural genetics study where most participants will likely not have an insomnia disorder.
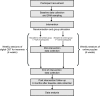
Methods and analysis: A two parallel-group randomised controlled trial is being conducted across three London universities. Female students (minimum age 18 years) enrolled on a psychology programme at one of the three sites were invited to participate. The target number of participants to be recruited is 240. Following baseline assessments, participants were randomly allocated to either the treatment group, where they received weekly sessions of digital CBT-I for 6 weeks, or the control group, where they completed an online puzzle each week for 6 weeks. Follow-up assessments have taken place mid-intervention (3 weeks) and end of intervention (6 weeks). A 6-month follow-up assessment will also occur. Primary outcomes will be assessed using descriptive statistics and effect size estimates for intervention effects. Secondary outcomes will be analysed using multivariate generalised estimating equation models.
Ethics and dissemination: The study received ethical approval from the Research Ethics and Integrity subcommittee, Goldsmiths, University of London (application reference: EA 1305). DNA sample collection for the BioResource received ethical approval from the NRES Committee South Central-Oxford (reference number: 15/SC/0388). The results of this work shall be published in peer-reviewed journals.
Trial registration number: NCT03062891; Results.
Keywords: adult psychiatry; psychiatry; sleep medicine.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: The position of AIL at the University of Oxford is funded by Big Health Ltd, the company behind the digital CBT-I programme evaluated in this study. CAE is the cofounder and CMO of Big Health Ltd and holds shares in Big Health Ltd. AMG has provided guidance and educational content for a freely available educational website focused on infant sleep. This website is partially supported by Johnson and Johnson, but they do not have any influence over content and do not advertise on it. AMG also contributes to BBC Focus Magazine.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical