Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis
- PMID: 29196621
- PMCID: PMC5711854
- DOI: 10.1038/s41467-017-01917-2
Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis
Abstract
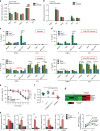
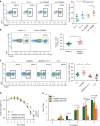
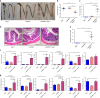
Inflammasomes are involved in gut homeostasis and inflammatory pathologies, but the role of NLRP3 inflammasome in these processes is not well understood. Cryopyrin-associated periodic syndrome (CAPS) patients with NLRP3 mutations have autoinflammation in skin, joints, and eyes, but not in the intestine. Here we show that the intestines of CAPS model mice carrying an Nlrp3 R258W mutation maintain homeostasis in the gut. Additionally, such mice are strongly resistant to experimental colitis and colorectal cancer; this is mainly through a remodelled gut microbiota with enhanced anti-inflammatory capacity due to increased induction of regulatory T cells (Tregs). Mechanistically, NLRP3R258W functions exclusively in the lamina propria mononuclear phagocytes to directly enhance IL-1β but not IL-18 secretion. Increased IL-1β boosts local antimicrobial peptides to facilitate microbiota remodelling. Our data show that NLRP3R258W-induced remodelling of the gut microbiota, induces local Tregs to maintain homeostasis and compensate for otherwise-detrimental intestinal inflammation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
A novel knock-in mouse model of cryopyrin-associated periodic syndromes with development of amyloidosis: Therapeutic efficacy of proton pump inhibitors.J Allergy Clin Immunol. 2020 Jan;145(1):368-378.e13. doi: 10.1016/j.jaci.2019.05.034. Epub 2019 Jun 10. J Allergy Clin Immunol. 2020. PMID: 31194989
-
Clinical and Molecular Phenotypes of Low-Penetrance Variants of NLRP3: Diagnostic and Therapeutic Challenges.Arthritis Rheumatol. 2017 Nov;69(11):2233-2240. doi: 10.1002/art.40208. Epub 2017 Oct 17. Arthritis Rheumatol. 2017. PMID: 28692792
-
Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies.J Clin Invest. 2013 Nov;123(11):4695-705. doi: 10.1172/JCI71543. J Clin Invest. 2013. PMID: 24084736 Free PMC article.
-
Low-frequency mosaicism in cryopyrin-associated periodic fever syndrome: mosaicism in systemic autoinflammatory diseases.Int Immunol. 2019 Sep 18;31(10):649-655. doi: 10.1093/intimm/dxz047. Int Immunol. 2019. PMID: 31185077 Review.
-
[Genetics of cryopyrin-associated periodic syndrome].Z Rheumatol. 2017 May;76(4):313-321. doi: 10.1007/s00393-017-0271-y. Z Rheumatol. 2017. PMID: 28197772 Review. German.
Cited by
-
Focus on the Role of NLRP3 Inflammasome in Diseases.Int J Mol Sci. 2020 Jun 13;21(12):4223. doi: 10.3390/ijms21124223. Int J Mol Sci. 2020. PMID: 32545788 Free PMC article. Review.
-
NLRP3 Inflammasome From Bench to Bedside: New Perspectives for Triple Negative Breast Cancer.Front Oncol. 2020 Sep 4;10:1587. doi: 10.3389/fonc.2020.01587. eCollection 2020. Front Oncol. 2020. PMID: 33014808 Free PMC article. Review.
-
NLRP3 Inflammasome and Inflammatory Bowel Disease.Front Immunol. 2019 Feb 28;10:276. doi: 10.3389/fimmu.2019.00276. eCollection 2019. Front Immunol. 2019. PMID: 30873162 Free PMC article. Review.
-
Intestinal microbiota regulates the gut-thyroid axis: the new dawn of improving Hashimoto thyroiditis.Clin Exp Med. 2024 Feb 22;24(1):39. doi: 10.1007/s10238-024-01304-4. Clin Exp Med. 2024. PMID: 38386169 Free PMC article. Review.
-
Exploring the glucose-lowering and anti-inflammatory immune mechanism of artemether by AMPK/mTOR pathway and microbiome based on multi-omics.Front Pharmacol. 2025 Feb 19;16:1520439. doi: 10.3389/fphar.2025.1520439. eCollection 2025. Front Pharmacol. 2025. PMID: 40046742 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

