The blood-brain barrier internalises Cryptococcus neoformans via the EphA2-tyrosine kinase receptor
- PMID: 29197141
- PMCID: PMC5836489
- DOI: 10.1111/cmi.12811
The blood-brain barrier internalises Cryptococcus neoformans via the EphA2-tyrosine kinase receptor
Abstract
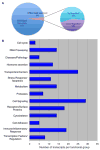
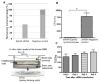
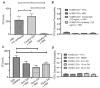
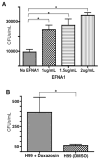
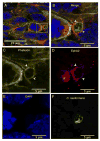
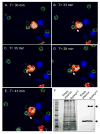
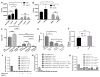
Cryptococcus neoformans is an opportunistic fungal pathogen that causes life-threatening meningitis most commonly in populations with impaired immunity. Here, we resolved the transcriptome of the human brain endothelium challenged with C. neoformans to establish whether C. neoformans invades the CNS by co-opting particular signalling pathways as a means to promote its own entry. Among the 5 major pathways targeted by C. neoformans, the EPH-EphrinA1 (EphA2) tyrosine kinase receptor-signalling pathway was examined further. Silencing the EphA2 receptor transcript in a human brain endothelial cell line or blocking EphA2 activity with an antibody or chemical inhibitor prevented transmigration of C. neoformans in an in vitro model of the blood-brain barrier (BBB). In contrast, treating brain endothelial cells with an EphA2 chemical agonist or an EphA2 ligand promoted greater migration of fungal cells across the BBB. C. neoformans activated the EPH-tyrosine kinase pathway through a CD44-dependent phosphorylation of EphA2, promoting clustering and internalisation of EphA2 receptors. Moreover, HEK293T cells expressing EphA2 revealed an association between EphA2 and C. neoformans that boosted internalisation of C. neoformans. Collectively, the results suggest that C. neoformans promotes EphA2 activity via CD44, and this in turn creates a permeable barrier that facilitates the migration of C. neoformans across the BBB.
Keywords: Cryptococcus neoformans; EphA2 receptor tyrosine kinase; blood-brain barrier; cytoskeleton remodelling; transcytosis of brain endothelial cells.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
-
- Grab DJ, Chakravorty SJ, van der Heyde H, Stins MF. How can microbial interactions with the blood-brain barrier modulate astroglial and neuronal function? Cell Microbiol. 2011;13:1470–1478. - PubMed
-
- Tauber SC, Eiffert H, Kellner S, Lugert R, Bunkowski S, et al. Fungal encephalitis in human autopsy cases is associated with extensive neuronal damage but only minimal repair. Neuropath Appl Neuro. 2014;40:610–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

