Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species
- PMID: 29197802
- PMCID: PMC5723279
- DOI: 10.1016/j.redox.2017.11.018
Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species
Abstract
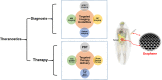
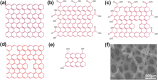
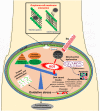
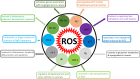
Graphene has a promising future in applications such as disease diagnosis, cancer therapy, drug/gene delivery, bio-imaging and antibacterial approaches owing to graphene's unique physical, chemical and mechanical properties alongside minimal toxicity to normal cells, and photo-stability. However, these unique features and bioavailability of graphene are fraught with uncertainties and concerns for environmental and occupational exposure. Changes in the physicochemical properties of graphene affect biological responses including reactive oxygen species (ROS) production. Lower production of ROS by currently available theranostic agents, e.g. magnetic nanoparticles, carbon nanotubes, gold nanostructures or polymeric nanoparticles, restricts their clinical application in cancer therapy. Oxidative stress induced by graphene accumulated in living organs is due to acellular factors which may affect physiological interactions between graphene and target tissues and cells. Acellular factors include particle size, shape, surface charge, surface containing functional groups, and light activation. Cellular responses such as mitochondrial respiration, graphene-cell interactions and pH of the medium are also determinants of ROS production. The mechanisms of ROS production by graphene and the role of ROS for cancer treatment, are poorly understood. The aim of this review is to set the theoretical basis for further research in developing graphene-based theranostic platforms.
Keywords: Bioimaging; Graphene; Photodynamic therapy; Reactive oxygen species; Singlet oxygen; Theranostics.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Toxicity and efficacy of carbon nanotubes and graphene: the utility of carbon-based nanoparticles in nanomedicine.Drug Metab Rev. 2014 May;46(2):232-46. doi: 10.3109/03602532.2014.883406. Epub 2014 Feb 10. Drug Metab Rev. 2014. PMID: 24506522 Review.
-
Bioapplications of graphene constructed functional nanomaterials.Chem Biol Interact. 2017 Jan 25;262:69-89. doi: 10.1016/j.cbi.2016.11.019. Epub 2016 Nov 20. Chem Biol Interact. 2017. PMID: 27876601 Review.
-
Lipid-Functionalized Graphene Loaded with hMnSOD for Selective Inhibition of Cancer Cells.ACS Appl Mater Interfaces. 2020 Mar 18;12(11):12407-12416. doi: 10.1021/acsami.9b20070. Epub 2020 Mar 6. ACS Appl Mater Interfaces. 2020. PMID: 32077682
-
Graphene-based nanomaterials as molecular imaging agents.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015 Nov-Dec;7(6):737-58. doi: 10.1002/wnan.1342. Epub 2015 Apr 6. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015. PMID: 25857851 Review.
-
Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress.ACS Nano. 2011 Sep 27;5(9):6971-80. doi: 10.1021/nn202451x. Epub 2011 Aug 24. ACS Nano. 2011. PMID: 21851105
Cited by
-
Enhancing the Efficiency of Mild-Temperature Photothermal Therapy for Cancer Assisting with Various Strategies.Pharmaceutics. 2022 Oct 24;14(11):2279. doi: 10.3390/pharmaceutics14112279. Pharmaceutics. 2022. PMID: 36365098 Free PMC article. Review.
-
Nanoparticle-Doped Hybrid Polyelectrolyte Microcapsules with Controlled Photoluminescence for Potential Bioimaging Applications.Polymers (Basel). 2021 Nov 24;13(23):4076. doi: 10.3390/polym13234076. Polymers (Basel). 2021. PMID: 34883579 Free PMC article.
-
Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells.Antioxidants (Basel). 2020 Mar 4;9(3):211. doi: 10.3390/antiox9030211. Antioxidants (Basel). 2020. PMID: 32143322 Free PMC article.
-
Evaluation of a biosensor-based graphene oxide-DNA nanohybrid for lung cancer.RSC Adv. 2023 Jan 17;13(4):2487-2500. doi: 10.1039/d2ra05808a. eCollection 2023 Jan 11. RSC Adv. 2023. PMID: 36741187 Free PMC article.
-
Surfactant Semiconductors as Trojan Horses in Cell-Membranes for On-Demand and Spatial Regulation of Oxidative Stress.Adv Healthc Mater. 2023 Apr;12(10):e2202290. doi: 10.1002/adhm.202202290. Epub 2023 Jan 13. Adv Healthc Mater. 2023. PMID: 36564363 Free PMC article.
References
-
- Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C., Bray F. International Agency for Research on Cancer; Lyon, France: 2013. Cancer Incidence and Mortality Worldwide: iarc CancerBase No. 11. (GLOBOCAN 2012 v1. 0, 2013)
-
- Stewart B.W.K.P., Wild C.P. World Cancer Report. 2014.
-
- Johnstone R.W., Ruefli A.A., Lowe S.W. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

