Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation
- PMID: 29197997
- PMCID: PMC6448958
- DOI: 10.1007/s00125-017-4509-7
Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation
Abstract
Aims/hypothesis: Sodium-glucose cotransporter 2 (SGLT2) inhibitors (SGLT2i) constitute a novel class of glucose-lowering (type 2) kidney-targeted agents. We recently reported that the SGLT2i empagliflozin (EMPA) reduced cardiac cytosolic Na+ ([Na+]c) and cytosolic Ca2+ ([Ca2+]c) concentrations through inhibition of Na+/H+ exchanger (NHE). Here, we examine (1) whether the SGLT2i dapagliflozin (DAPA) and canagliflozin (CANA) also inhibit NHE and reduce [Na+]c; (2) a structural model for the interaction of SGLT2i to NHE; (3) to what extent SGLT2i affect the haemodynamic and metabolic performance of isolated hearts of healthy mice.
Methods: Cardiac NHE activity and [Na+]c in mouse cardiomyocytes were measured in the presence of clinically relevant concentrations of EMPA (1 μmol/l), DAPA (1 μmol/l), CANA (3 μmol/l) or vehicle. NHE docking simulation studies were applied to explore potential binding sites for SGTL2i. Constant-flow Langendorff-perfused mouse hearts were subjected to SGLT2i for 30 min, and cardiovascular function, O2 consumption and energetics (phosphocreatine (PCr)/ATP) were determined.
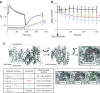
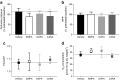
Results: EMPA, DAPA and CANA inhibited NHE activity (measured through low pH recovery after NH4+ pulse: EMPA 6.69 ± 0.09, DAPA 6.77 ± 0.12 and CANA 6.80 ± 0.18 vs vehicle 7.09 ± 0.09; p < 0.001 for all three comparisons) and reduced [Na+]c (in mmol/l: EMPA 10.0 ± 0.5, DAPA 10.7 ± 0.7 and CANA 11.0 ± 0.9 vs vehicle 12.7 ± 0.7; p < 0.001). Docking studies provided high binding affinity of all three SGLT2i with the extracellular Na+-binding site of NHE. EMPA and CANA, but not DAPA, induced coronary vasodilation of the intact heart. PCr/ATP remained unaffected.
Conclusions/interpretation: EMPA, DAPA and CANA directly inhibit cardiac NHE flux and reduce [Na+]c, possibly by binding with the Na+-binding site of NHE-1. Furthermore, EMPA and CANA affect the healthy heart by inducing vasodilation. The [Na+]c-lowering class effect of SGLT2i is a potential approach to combat elevated [Na+]c that is known to occur in heart failure and diabetes.
Keywords: Cardiac; Diabetes; Heart failure; Na+/H+ exchanger; SGLT2i; Sodium; Vasodilation.
Conflict of interest statement
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
CAS, JWTF, MWH and NCW contributed to the analysis planning and interpretation of data, and reviewed and edited the manuscript. MJ and AK contributed to the acquisition of data and reviewed the manuscript. LU, AB, BB, RC and CJZ contributed to the conception and design, acquisition of data, and analysis and interpretation of data, and drafting or revising the critical intellectual content of the manuscript. All authors are responsible for the content and approved the final version. CJZ is guarantor of this work.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

