Hypoxic Induction of Vasorin Regulates Notch1 Turnover to Maintain Glioma Stem-like Cells
- PMID: 29198941
- PMCID: PMC5756127
- DOI: 10.1016/j.stem.2017.10.005
Hypoxic Induction of Vasorin Regulates Notch1 Turnover to Maintain Glioma Stem-like Cells
Abstract
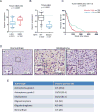
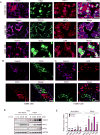
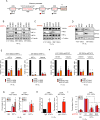
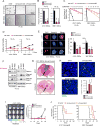
Tumor hypoxia is associated with poor patient survival and is a characteristic of glioblastoma. Notch signaling is implicated in maintaining glioma stem-like cells (GSCs) within the hypoxic niche, although the molecular mechanisms linking hypoxia to Notch activation have not been clearly delineated. Here we show that Vasorin is a critical link between hypoxia and Notch signaling in GSCs. Vasorin is preferentially induced in GSCs by a HIF1α/STAT3 co-activator complex and stabilizes Notch1 protein at the cell membrane. This interaction prevents Numb from binding Notch1, rescuing it from Numb-mediated lysosomal degradation. Thus, Vasorin acts as a switch to augment Notch signaling under hypoxic conditions. Vasorin promotes tumor growth and reduces survival in mouse models of glioblastoma, and its expression correlates with increased aggression of human gliomas. These findings provide mechanistic insights into how hypoxia promotes Notch signaling in glioma and identify Vasorin as a potential therapeutic target.
Keywords: HIF1; Notch; Numb; STAT3; Vasorin; glioblastoma; glioma; glioma stem-like cells; hypoxia; pseudohypoxia.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

Comment in
-
The STAT3 and hypoxia pathways converge on Vasorin to promote stemness and glioblastoma tumorigenesis through Notch1 stabilization.Stem Cell Investig. 2018 Oct 22;5:35. doi: 10.21037/sci.2018.10.01. eCollection 2018. Stem Cell Investig. 2018. PMID: 30498746 Free PMC article. No abstract available.
-
Picture of glioma stem cells has become a Notch brighter.Stem Cell Investig. 2018 Nov 21;5:42. doi: 10.21037/sci.2018.11.02. eCollection 2018. Stem Cell Investig. 2018. PMID: 30596082 Free PMC article. No abstract available.
References
-
- Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nature reviews Drug discovery. 2014;13:357–378. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

