Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry
- PMID: 29199008
- PMCID: PMC5818302
- DOI: 10.1016/j.dental.2017.11.020
Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry
Abstract
Photopolymerized hydrogels, such as gelatin methacryloyl (GelMA), have desirable biological and mechanical characteristics for a range of tissue engineering applications.
Objective: This study aimed to optimize a new method to photopolymerize GelMA using a dental curing light (DL).
Methods: Lithium acylphosphinate photo-initiator (LAP, 0.05, 0.067, 0.1% w/v) was evaluated for its ability to polymerize GelMA hydrogel precursors (10% w/v) encapsulated with odontoblast-like cells (OD21). Different irradiances (1650, 2300 and 3700mW/cm2) and photo-curing times (5-20s) were tested, and compared against the parameters typically used in UV light photopolymerization (45mW/cm2, 0.1% w/v Irgacure 2959 as photoinitiator). Physical and mechanical properties of the photopolymerized GelMA hydrogels were determined. Cell viability was assessed using a live and dead assay kit.
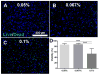
Results: Comparing DL and UV polymerization methods, the DL method photopolymerized GelMA precursor faster and presented larger pore size than the UV polymerization method. The live and dead assay showed more than 80% of cells were viable when hydrogels were photopolymerized with the different DL irradiances. However, the cell viability decreased when the exposure time was increased to 20s using the 1650mW/cm2 intensity, and when the LAP concentration was increased from 0.05 to 0.1%. Both DL and UV photocrosslinked hydrogels supported a high percentage of cell viability and enabled fabrication of micropatterns using a photolithography microfabrication technique.
Significance: The proposed method to photopolymerize GelMA cell-laden hydrogels using a dental curing light is effective and represents an important step towards the establishment of chair-side procedures in regenerative dentistry.
Keywords: Bioengineering; Biomedical and dental materials; Endodontics; Hydrogel; Odontoblast; Regenerative medicine; Visible light.
Copyright © 2017 The Academy of Dental Materials. Published by Elsevier Ltd. All rights reserved.
Figures







Similar articles
-
Gelatin Methacryloyl-Riboflavin (GelMA-RF) Hydrogels for Bone Regeneration.Int J Mol Sci. 2021 Feb 6;22(4):1635. doi: 10.3390/ijms22041635. Int J Mol Sci. 2021. PMID: 33561941 Free PMC article.
-
Photopolymerization of cell-encapsulating hydrogels: crosslinking efficiency versus cytotoxicity.Acta Biomater. 2012 May;8(5):1838-48. doi: 10.1016/j.actbio.2011.12.034. Epub 2012 Jan 13. Acta Biomater. 2012. PMID: 22285429
-
Engineering Microvascular Networks in LED Light-Cured Cell-Laden Hydrogels.ACS Biomater Sci Eng. 2018 Jul 9;4(7):2563-2570. doi: 10.1021/acsbiomaterials.8b00502. Epub 2018 Jun 26. ACS Biomater Sci Eng. 2018. PMID: 33435119
-
Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels.Biomaterials. 2015 Dec;73:254-71. doi: 10.1016/j.biomaterials.2015.08.045. Epub 2015 Aug 28. Biomaterials. 2015. PMID: 26414409 Free PMC article. Review.
-
Recent advances in photo-crosslinkable hydrogels for biomedical applications.Biotechniques. 2019 Jan;66(1):40-53. doi: 10.2144/btn-2018-0083. Biotechniques. 2019. PMID: 30730212 Review.
Cited by
-
Cell-scaffold interactions in tissue engineering for oral and craniofacial reconstruction.Bioact Mater. 2022 Nov 8;23:16-44. doi: 10.1016/j.bioactmat.2022.10.029. eCollection 2023 May. Bioact Mater. 2022. PMID: 36406245 Free PMC article.
-
Micropatterned hydrogels and cell alignment enhance the odontogenic potential of stem cells from apical papilla in-vitro.Dent Mater. 2020 Jan;36(1):88-96. doi: 10.1016/j.dental.2019.10.013. Epub 2019 Nov 25. Dent Mater. 2020. PMID: 31780101 Free PMC article.
-
Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: an Effective Strategy for Tissue Engineering.Stem Cell Rev Rep. 2019 Oct;15(5):664-679. doi: 10.1007/s12015-019-09893-4. Stem Cell Rev Rep. 2019. PMID: 31154619 Review.
-
Gelatin Methacryloyl-Riboflavin (GelMA-RF) Hydrogels for Bone Regeneration.Int J Mol Sci. 2021 Feb 6;22(4):1635. doi: 10.3390/ijms22041635. Int J Mol Sci. 2021. PMID: 33561941 Free PMC article.
-
Nanoscale mineralization of cell-laden methacrylated gelatin hydrogels using calcium carbonate-calcium citrate core-shell microparticles.J Mater Chem B. 2021 Dec 1;9(46):9583-9593. doi: 10.1039/d1tb01673c. J Mater Chem B. 2021. PMID: 34779469 Free PMC article.
References
-
- Petrie Aronin CE, Kuhn NZ, Tuan RS. Tissue Engineering and Selection of Cells. In: Paul D Editor-in-Chief, editor. Comprehensive Biomaterials. Oxford: Elsevier; 2011. pp. 81–93.
-
- Bidarra SJ, Barrias CC, Granja PL. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014;10:1646–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

