Choroidal Changes After Suprachoroidal Injection of Triamcinolone Acetonide in Eyes With Macular Edema Secondary to Retinal Vein Occlusion
- PMID: 29199012
- PMCID: PMC5805638
- DOI: 10.1016/j.ajo.2017.11.020
Choroidal Changes After Suprachoroidal Injection of Triamcinolone Acetonide in Eyes With Macular Edema Secondary to Retinal Vein Occlusion
Abstract
Purpose: To evaluate choroidal and suprachoroidal changes following suprachoroidal injection of triamcinolone acetonide injectable suspension (CLS-TA), in eyes with macular edema due to retinal vein occlusion (RVO).
Design: Prospective cohort study within a randomized, controlled phase 2 clinical trial.
Methods: Enhanced depth imaging optical coherence tomography (EDI-OCT) images were analyzed from 38 eyes of 38 treatment-naïve patients with macular edema due to RVO, enrolled in the prospective Suprachoroidal Injection of Triamcinolone Acetonide with Intravitreal Aflibercept in Subjects with Macular Edema Due to Retinal Vein Occlusion (TANZANITE) study who received either a suprachoroidal injection of CLS-TA with an intravitreal injection of aflibercept (combination arm) or only an intravitreal injection of aflibercept (monotherapy arm), followed by monthly intravitreal aflibercept injections in both arms based on pro re nata criteria.
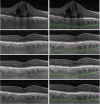
Results: Macular choroidal thickness measured to the outer choroidal vessel lumen (vascular choroidal thickness, VCT), outer choroid stroma (stromal choroidal thickness, SCT), or inner scleral border (total choroidal thickness, TCT) showed no significant changes over 3 months in both study arms (P = .231-.342). Eyes that received combination therapy showed a trend toward thickening of the suprachoroidal space (SCS) compared with monotherapy alone (13.4 μm vs 5.3 μm at 3 months; P = .077). In the 15 eyes that demonstrated a visible SCS at baseline, the SCS expanded significantly after suprachoroidal CLS-TA injection (16.2 μm to 27.8 μm at 3 months; P = .033).
Conclusions: Suprachoroidal injection of CLS-TA does not alter choroidal thickness in eyes with macular edema due to RVO, but may result in expansion of the SCS.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
AW: none
VV: none
GN: employee, patents, and equity at Clearside
RD: equity and employee relationship at EyeKor Inc.
DC: none
SF: patents in OCT imaging and analysis
GY: grants from Alcon, ARVO Foundation, E Matilda Ziegler Foundation, Genentech and personal fees for consultancy from Alimera, Allergan, Carl Zeiss Meditec, and Southern California Desert Retina.
Figures



Comment in
-
Choroidal Changes After Suprachoroidal Injection of Triamcinolone in Eyes With Macular Edema Secondary to Retinal Vein Occlusion.Am J Ophthalmol. 2018 May;189:177-178. doi: 10.1016/j.ajo.2018.02.022. Epub 2018 Mar 21. Am J Ophthalmol. 2018. PMID: 29573779 No abstract available.
-
Reply.Am J Ophthalmol. 2018 May;189:178. doi: 10.1016/j.ajo.2018.03.002. Epub 2018 Mar 22. Am J Ophthalmol. 2018. PMID: 29576184 No abstract available.
References
-
- Ho M, Liu DT, Lam DS, Jonas JB. Retinal Vein Occlusions, From Basics to the Latest Treatment. Retina. 2016;36(3):432–48. - PubMed
-
- Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114(10):1243–7. - PubMed
-
- Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56(4):281–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

