Intussusception hospitalizations before rotavirus vaccine introduction: Retrospective data from two referral hospitals in Tamil Nadu, India
- PMID: 29199044
- PMCID: PMC6290388
- DOI: 10.1016/j.vaccine.2017.11.043
Intussusception hospitalizations before rotavirus vaccine introduction: Retrospective data from two referral hospitals in Tamil Nadu, India
Abstract
Background: The indigenous oral rotavirus vaccine Rotavac® was introduced into the public immunization system in India in 2016 and will be expanded in phases. This data will describe the epidemiology of intussusception in India in absence of rotavirus vaccination and will help in setting up or designing a safety monitoring system.
Methods: Medical records of intussusception cases between 2013 and 2016 in two major referral hospitals in Tamil Nadu, India were reviewed, and data on clinical presentation and management and outcome were collated.
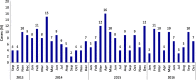
Results: A total of 284 cases of intussusception were diagnosed and managed at the two centers of which 280/284 could be classified as level 1 by the Brighton criteria. Median age at presentation was 8 months (Inter Quartile Range, IQR 6-17.2) with a male to female ratio of 2.1:1. Over half (57.7%) required surgical intervention while the rest underwent non-surgical or conservative management.
Conclusions: Retrospective data from referral hospitals is sufficient to classify cases of intussusception by the Brighton criteria. These baseline data will be useful for monitoring when rotavirus vaccination is introduced.
Keywords: Children; Intussusception; Retrospective surveillance; Rotavirus vaccine.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- National and Regional Rotavirus Vaccine Introductions – ROTA Council n.d. <http://rotacouncil.org/toolkit/national-and-regional-rotavirus-introduct...> [accessed February 21, 2017].
-
- Shri J P Nadda launches Rotavirus vaccine as part of Universal Immunization Programme; terms it a “historic moment” n.d. <http://pib.nic.in/newsite/PrintRelease.aspx?relid=138342> [accessed August 4, 2016].
-
- John J., Sarkar R., Muliyil J., Bhandari N., Bhan M.K., Kang G. Rotavirus gastroenteritis in India, 2011–2013: revised estimates of disease burden and potential impact of vaccines. Vaccine. 2014;32:A5–A9. - PubMed
-
- Patel M.M., López-Collada V.R., Bulhões M.M., De Oliveira L.H., Márquez A.B., Flannery B. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–2292. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical