Theta Oscillations in the Human Medial Temporal Lobe during Real-World Ambulatory Movement
- PMID: 29199073
- PMCID: PMC5937848
- DOI: 10.1016/j.cub.2017.10.062
Theta Oscillations in the Human Medial Temporal Lobe during Real-World Ambulatory Movement
Abstract
The theta rhythm-a slow (6-12 Hz) oscillatory component of the local field potential-plays a critical role in spatial navigation and memory by coordinating the activity of neuronal ensembles within the medial temporal lobe (MTL). Although theta has been extensively studied in freely moving rodents, its presence in humans has been elusive and primarily investigated in stationary subjects. Here we used a unique clinical opportunity to examine theta within the human MTL during untethered, real-world ambulatory movement. We recorded intracranial electroencephalographic activity from participants chronically implanted with the wireless NeuroPace responsive neurostimulator (RNS) and tracked their motion with sub-millimeter precision. Our data revealed that movement-related theta oscillations indeed exist in humans, such that theta power is significantly higher during movement than immobility. Unlike in rodents, however, theta occurs in short bouts, with average durations of ∼400 ms, which are more prevalent during fast versus slow movements. In a rare opportunity to study a congenitally blind participant, we found that both the prevalence and duration of theta bouts were increased relative to the sighted participants. These results provide critical support for conserved neurobiological characteristics of theta oscillations during ambulatory spatial navigation, while highlighting some fundamental differences across species in these oscillations between humans and rodents.
Keywords: MTL; NeuroPace responsive neurostimulator; human; medial temporal lobe; navigation; theta oscillations; wireless intracranial recordings.
Published by Elsevier Ltd.
Figures




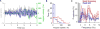

Comment in
-
Brain Rhythms: Higher-Frequency Theta Oscillations Make Sense in Moving Humans.Curr Biol. 2018 Jan 22;28(2):R70-R72. doi: 10.1016/j.cub.2017.11.045. Curr Biol. 2018. PMID: 29374447 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

