Antibiotic-Induced Changes to the Host Metabolic Environment Inhibit Drug Efficacy and Alter Immune Function
- PMID: 29199098
- PMCID: PMC5730482
- DOI: 10.1016/j.chom.2017.10.020
Antibiotic-Induced Changes to the Host Metabolic Environment Inhibit Drug Efficacy and Alter Immune Function
Abstract
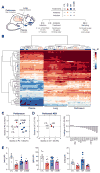
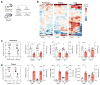
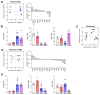
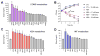
Bactericidal antibiotics alter microbial metabolism as part of their lethality and can damage mitochondria in mammalian cells. In addition, antibiotic susceptibility is sensitive to extracellular metabolites, but it remains unknown whether metabolites present at an infection site can affect either treatment efficacy or immune function. Here, we quantify local metabolic changes in the host microenvironment following antibiotic treatment for a peritoneal Escherichia coli infection. Antibiotic treatment elicits microbiome-independent changes in local metabolites, but not those distal to the infection site, by acting directly on host cells. The metabolites induced during treatment, such as AMP, reduce antibiotic efficacy and enhance phagocytic killing. Moreover, antibiotic treatment impairs immune function by inhibiting respiratory activity in immune cells. Collectively, these results highlight the immunomodulatory potential of antibiotics and reveal the local metabolic microenvironment to be an important determinant of infection resolution.
Keywords: LC-MS/MS; antibiotic; germ-free; immunomodulation; metabolic environment; metabolomics; phagocytosis; respiration; systems biology.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
-
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl 1):5–16. - PubMed
-
- Anuforom O, Wallace GR, Piddock LV. The immune response and antibacterial therapy. Med Microbiol Immunol. 2015;204:151–159. - PubMed
-
- Ballabio D, Consonni V. Classification tools in chemistry. Part 1: linear models. PLS-DA. Anal Methods-Uk. 2013;5:3790–3798.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

