The renaissance of complement therapeutics
- PMID: 29199277
- PMCID: PMC5805379
- DOI: 10.1038/nrneph.2017.156
The renaissance of complement therapeutics
Abstract
The increasing number of clinical conditions that involve a pathological contribution from the complement system - many of which affect the kidneys - has spurred a regained interest in therapeutic options to modulate this host defence pathway. Molecular insight, technological advances, and the first decade of clinical experience with the complement-specific drug eculizumab, have contributed to a growing confidence in therapeutic complement inhibition. More than 20 candidate drugs that target various stages of the complement cascade are currently being evaluated in clinical trials, and additional agents are in preclinical development. Such diversity is clearly needed in view of the complex and distinct involvement of complement in a wide range of clinical conditions, including rare kidney disorders, transplant rejection and haemodialysis-induced inflammation. The existing drugs cannot be applied to all complement-driven diseases, and each indication has to be assessed individually. Alongside considerations concerning optimal points of intervention and economic factors, patient stratification will become essential to identify the best complement-specific therapy for each individual patient. This Review provides an overview of the therapeutic concepts, targets and candidate drugs, summarizes insights from clinical trials, and reflects on existing challenges for the development of complement therapeutics for kidney diseases and beyond.
Figures
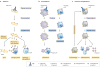


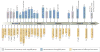
References
-
- Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. - PubMed
-
- Center for Drug Evaluation and Research. Application number: 125166, approval letter. Food and Drug Administration. 2007 https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/125166s0000_APPROV.
-
- Center for Drug Evaluation and Research. Application number: 125166s172, approval letter. Food and Drug Administration. 2011 https://www.accessdata.fda.gov/drugsatfda_docs/bla/2011/125166Orig1s172-....
-
- Fakhouri F, Fremeaux-Bacchi V. Thrombotic microangiopathy: eculizumab for atypical haemolytic uraemic syndrome: what next? Nat Rev Nephrol. 2013;9:495–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

