Antifibrinolytic Therapy and Perioperative Considerations
- PMID: 29200009
- PMCID: PMC5811331
- DOI: 10.1097/ALN.0000000000001997
Antifibrinolytic Therapy and Perioperative Considerations
Abstract
Fibrinolysis is a physiologic component of hemostasis that functions to limit clot formation. However, after trauma or surgery, excessive fibrinolysis may contribute to coagulopathy, bleeding, and inflammatory responses. Antifibrinolytic agents are increasingly used to reduce bleeding, allogeneic blood administration, and adverse clinical outcomes. Tranexamic acid is the agent most extensively studied and used in most countries. This review will explore the role of fibrinolysis as a pathologic mechanism, review the different pharmacologic agents used to inhibit fibrinolysis, and focus on the role of tranexamic acid as a therapeutic agent to reduce bleeding in patients after surgery and trauma.
Conflict of interest statement
Conflicts of interest:
JHL- serves on steering committees for Boehringer Ingelheim, CSL Behring, Grifols, Instrumentation Labs, and on advisory committees for Leading Biosciences, Octapharma, Pfizer, and Portola
AK- none
QJK- none
TJM- steering committee Portola, consultant CSL Behring
NSK- consultant Bayer, CSL Behring; research support: Baxalta US Inc
Figures

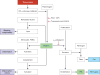
References
-
- Longstaff C, Kolev K. Basic mechanisms and regulation of fibrinolysis. J Thromb Haemost. 2015;13(Suppl 1):S98–105. - PubMed
-
- Wiman B, Collen D. Molecular mechanism of physiological fibrinolysis. Nature. 1978;272:549–50. - PubMed
-
- Ilich A, Bokarev I, Key NS. Global assays of fibrinolysis. Int J Lab Hematol. 2017 - PubMed
-
- Levy JH. Antifibrinolytic therapy: new data and new concepts. Lancet. 2010;376:3–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

