Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function
- PMID: 29200204
- PMCID: PMC5760280
- DOI: 10.1038/nm.4447
Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function
Abstract
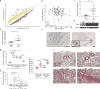
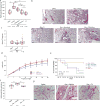
Thyroid hormone (TH) is critical for the maintenance of cellular homeostasis during stress responses, but its role in lung fibrosis is unknown. Here we found that the activity and expression of iodothyronine deiodinase 2 (DIO2), an enzyme that activates TH, were higher in lungs from patients with idiopathic pulmonary fibrosis than in control individuals and were correlated with disease severity. We also found that Dio2-knockout mice exhibited enhanced bleomycin-induced lung fibrosis. Aerosolized TH delivery increased survival and resolved fibrosis in two models of pulmonary fibrosis in mice (intratracheal bleomycin and inducible TGF-β1). Sobetirome, a TH mimetic, also blunted bleomycin-induced lung fibrosis. After bleomycin-induced injury, TH promoted mitochondrial biogenesis, improved mitochondrial bioenergetics and attenuated mitochondria-regulated apoptosis in alveolar epithelial cells both in vivo and in vitro. TH did not blunt fibrosis in Ppargc1a- or Pink1-knockout mice, suggesting dependence on these pathways. We conclude that the antifibrotic properties of TH are associated with protection of alveolar epithelial cells and restoration of mitochondrial function and that TH may thus represent a potential therapy for pulmonary fibrosis.
Conflict of interest statement
All other authors declare no additional competing interests.
Figures




Comment in
-
Thyroid function: Thyroid hormone therapy resolves pulmonary fibrosis in mice.Nat Rev Endocrinol. 2018 Feb;14(2):64. doi: 10.1038/nrendo.2017.172. Epub 2017 Dec 22. Nat Rev Endocrinol. 2018. PMID: 29286044 No abstract available.
-
New Applications of Old Drugs as Novel Therapies in Idiopathic Pulmonary Fibrosis. Metformin, Hydroxychloroquine, and Thyroid Hormone.Am J Respir Crit Care Med. 2019 Jun 15;199(12):1561-1563. doi: 10.1164/rccm.201809-1700RR. Am J Respir Crit Care Med. 2019. PMID: 30822095 Free PMC article. No abstract available.
-
Recent Insights into the Involvement of Novel Transcription Factors, The Microbiome, and Dysregulated Cellular Metabolism in Pulmonary Fibrosis Pathogenesis.Am J Respir Cell Mol Biol. 2019 Nov;61(5):653-655. doi: 10.1165/rcmb.2019-0196RO. Am J Respir Cell Mol Biol. 2019. PMID: 31265328 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- RC2 HL101715/HL/NHLBI NIH HHS/United States
- R01 HL127349/HL/NHLBI NIH HHS/United States
- UH3 HL123886/HL/NHLBI NIH HHS/United States
- T32 HL007778/HL/NHLBI NIH HHS/United States
- R01 HL109233/HL/NHLBI NIH HHS/United States
- R01 DK052798/DK/NIDDK NIH HHS/United States
- R01 HL095397/HL/NHLBI NIH HHS/United States
- R01 HL125850/HL/NHLBI NIH HHS/United States
- R01 DK065055/DK/NIDDK NIH HHS/United States
- R01 HL138396/HL/NHLBI NIH HHS/United States
- UH2 HL123886/HL/NHLBI NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

