Amyloid-β plaques enhance Alzheimer's brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation
- PMID: 29200205
- PMCID: PMC5760353
- DOI: 10.1038/nm.4443
Amyloid-β plaques enhance Alzheimer's brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation
Abstract
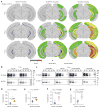
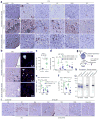
Alzheimer's disease (AD) is characterized by extracellular amyloid-β (Aβ) plaques and intracellular tau inclusions. However, the exact mechanistic link between these two AD lesions remains enigmatic. Through injection of human AD-brain-derived pathological tau (AD-tau) into Aβ plaque-bearing mouse models that do not overexpress tau, we recapitulated the formation of three major types of AD-relevant tau pathologies: tau aggregates in dystrophic neurites surrounding Aβ plaques (NP tau), AD-like neurofibrillary tangles (NFTs) and neuropil threads (NTs). These distinct tau pathologies have different temporal onsets and functional consequences on neural activity and behavior. Notably, we found that Aβ plaques created a unique environment that facilitated the rapid amplification of proteopathic AD-tau seeds into large tau aggregates, initially appearing as NP tau, which was followed by the formation and spread of NFTs and NTs, likely through secondary seeding events. Our study provides insights into a new multistep mechanism underlying Aβ plaque-associated tau pathogenesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
-
- Gómez-Isla T, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. - PubMed
-
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–384. - PubMed
-
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

