Long-term sensorimotor adaptation in the ocular following system of primates
- PMID: 29200430
- PMCID: PMC5714349
- DOI: 10.1371/journal.pone.0189030
Long-term sensorimotor adaptation in the ocular following system of primates
Abstract
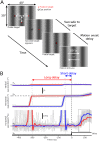
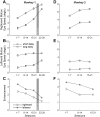
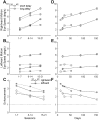
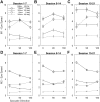
The sudden movement of a wide-field image leads to a reflexive eye tracking response referred to as short-latency ocular following. If the image motion occurs soon after a saccade the initial speed of the ocular following is enhanced, a phenomenon known as post-saccadic enhancement. We show in macaque monkeys that repeated exposure to the same stimulus regime over a period of months leads to progressive increases in the initial speeds of ocular following. The improvement in tracking speed occurs for ocular following with and without a prior saccade. As a result of the improvement in ocular following speeds, the influence of post-saccadic enhancement wanes with increasing levels of training. The improvement in ocular following speed following repeated exposure to the same oculomotor task represents a novel form of sensori-motor learning in the context of a reflexive movement.
Conflict of interest statement
Figures
Similar articles
-
Short-term adaptation of saccades does not affect smooth pursuit eye movement initiation.J Vis. 2017 Aug 1;17(9):19. doi: 10.1167/17.9.19. J Vis. 2017. PMID: 28837965
-
The initiation of smooth pursuit eye movements and saccades in normal subjects and in "express-saccade makers".Exp Brain Res. 2002 Jun;144(3):373-84. doi: 10.1007/s00221-002-1059-z. Epub 2002 Apr 13. Exp Brain Res. 2002. PMID: 12021819
-
Enhanced motion sensitivity follows saccadic suppression in the superior temporal sulcus of the macaque cortex.Cereb Cortex. 2007 May;17(5):1129-38. doi: 10.1093/cercor/bhl022. Epub 2006 Jun 19. Cereb Cortex. 2007. PMID: 16785254
-
Saccade adaptation as a model of flexible and general motor learning.Exp Eye Res. 2013 Sep;114:6-15. doi: 10.1016/j.exer.2013.04.001. Epub 2013 Apr 15. Exp Eye Res. 2013. PMID: 23597598 Free PMC article. Review.
-
The characteristics and neuronal substrate of saccadic eye movement plasticity.Prog Neurobiol. 2004 Jan;72(1):27-53. doi: 10.1016/j.pneurobio.2003.12.002. Prog Neurobiol. 2004. PMID: 15019175 Review.
Cited by
-
Smooth Pursuit Eye Movement of Monkeys Naive to Laboratory Setups With Pictures and Artificial Stimuli.Front Syst Neurosci. 2018 Apr 17;12:15. doi: 10.3389/fnsys.2018.00015. eCollection 2018. Front Syst Neurosci. 2018. PMID: 29719503 Free PMC article.
-
Metrics of two-dimensional smooth pursuit are diverse across participants and stable across days.J Vis. 2025 Feb 3;25(2):5. doi: 10.1167/jov.25.2.5. J Vis. 2025. PMID: 39903185 Free PMC article.
-
Sensorimotor-linked reward modulates smooth pursuit eye movements in monkeys.Front Neurosci. 2024 Jan 9;17:1297914. doi: 10.3389/fnins.2023.1297914. eCollection 2023. Front Neurosci. 2024. PMID: 38264498 Free PMC article.
-
The Speed of Neural Visual Motion Perception and Processing Determines the Visuomotor Reaction Time of Young Elite Table Tennis Athletes.Front Behav Neurosci. 2019 Jul 19;13:165. doi: 10.3389/fnbeh.2019.00165. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31379535 Free PMC article.
-
Ocular following responses of the marmoset monkey are dependent on postsaccadic delay, spatiotemporal frequency, and saccade direction.J Neurophysiol. 2023 Jul 1;130(1):189-198. doi: 10.1152/jn.00126.2023. Epub 2023 Jun 28. J Neurophysiol. 2023. PMID: 37377195 Free PMC article.
References
-
- Miles FA, Kawano K, Optican LM.Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of visual input. J Neurophysiol 1986;56: 1321–1354. - PubMed
-
- Gellman RS, Carl JR, Miles FA. Short latency ocular-following responses in man. Vis Neurosci 1990;5: 107–122. - PubMed
-
- Kawano K, Miles FA. Short-latency ocular following responses of monkey. II. Dependence on a prior saccadic eye movement. J Neurophysiol 1986;56: 1355–1380. - PubMed
-
- Ibbotson MR, Crowder NA, Cloherty SL, Price NS, Mustari MJ. Saccadic modulation of neural responses: possible roles in saccadic suppression, enhancement, and time compression. J Neurosci 2008;28: 10952–10960. doi: 10.1523/JNEUROSCI.3950-08.2008 - DOI - PMC - PubMed
-
- Cloherty SL, Mustari MJ, Rosa MG, Ibbotson MR. Effects of saccades on visual processing in primate MSTd. Vision Res 2010;50: 2683–2691. doi: 10.1016/j.visres.2010.08.020 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

