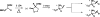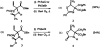Synthesis of Higher α-Chlorovinyl and α-Bromovinyl Amino Acids: The Amino Protecting Group Determines the Reaction Course
- PMID: 29200438
- PMCID: PMC5708561
- DOI: 10.1016/0040-4039(96)00832-5
Synthesis of Higher α-Chlorovinyl and α-Bromovinyl Amino Acids: The Amino Protecting Group Determines the Reaction Course
Abstract
N-Trifluoroacetyl α-vinyl amino esters are smoothly converted to the corresponding α-chlorovinyl or α-bromovinyl amino esters through the agency of phenyselenyl chloride or phenylselenyl bromide, respectively, followed by oxidation and pyrolysis. Exclusively the (E)-extemal halovinyl isomer and the internal halovinyl isomer are observed. The amino protecting group is a critical determinant of the reaction course (alkene addition vs. 5-exo-trig-like cyclization).
References
-
- Abeles RH, Maycock AL. Acc Chem Res. 1976;9:313–319.
-
- Thornberry NA, Bull HG, Taub D, Greenlee WJ, Patchett AA, Cordes EH. J Am Chem Soc. 1987;109:7543–7544.
- Thomberry NA, Bull HG, Taub D, Wilson KE, Gimenez-Gallego G, Rosegay A, Soderman DD, Patchett AA. J Biol Chem. 1991;266:21657–21665. - PubMed
- Patchett AA, Taub D, Weissberger B, Valiant ME, Gadebusch H, Thomberry NA, Bull HG. Antimicrob Agents and Chemother. 1988;32:319–323. - PMC - PubMed
-
- Xu Y, Abeles RH. Biochem. 1993;32:806–811. - PubMed
-
- Silverman RB, Bichler KA, Leon AJ. J Am Chem Soc. 1996;118:1241–1252.
- Silverman RB, Bichler KA, Leon AJ. Ibid. 1996;118:1253–1261.
- Kolb M, Barth J, Heydt J-G, Jung MJ. J Med Chem. 1987;30:267–272. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources