Simvastatin/Ezetimibe Therapy for Recalcitrant Alopecia Areata: An Open Prospective Study of 14 Patients
- PMID: 29200765
- PMCID: PMC5705358
- DOI: 10.5021/ad.2017.29.6.755
Simvastatin/Ezetimibe Therapy for Recalcitrant Alopecia Areata: An Open Prospective Study of 14 Patients
Abstract
Background: Simvastatin belongs to the statin family, whose members have immunomodulatory activities. Ezetimibe have synergetic effects when co-administered with simvastatin. In several case reports, alopecia totalis and alopecia universalis were successfully treated with simvastatin/ezetimibe, suggesting that this combination could be a new efficient therapy for recalcitrant alopecia areata (AA).
Objective: To verify the efficacy of the simvastatin/ezetimibe combination therapy for recalcitrant AA and investigate the relationship between various treatment responses and prognostic factors.
Methods: This prospective open study was performed in patients with recalcitrant AA with the bald surface exceeding 75%. All patients took simvastatin (40 mg) and ezetimibe (10 mg) daily. The extent of hair regrowth expressed as percentage of the bald area was used to evaluate the effectiveness of the therapy.
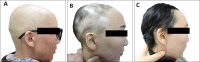
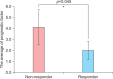
Results: Of 14 enrolled patients, 4 patients (28.6%) were judged as responders showing regrowth of 30% to 80% after 3 months of treatment. The mean age of onset in non-responders was significantly lower than in responders. The total score of prognostic factors, calculated as a sum of factors related to poor prognosis, was much lower in responders than in non-responders.
Conclusion: The remission rate in this study was unsatisfactory. However, since the recruited patients had not responded to any other treatments for AA, simvastatin/ezetimibe can still be considered as an alternative treatment for recalcitrant AA. The total scores of the prognostic factors were statistically different between responders and non-responders. These results can be used to predict the outcome of treatment with simvastatin/ezetimibe and anticipate prognosis.
Keywords: Alopecia areata; Ezetimibe; Prognostic factor; Simvastatin.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors have nothing to disclose.
Figures

References
-
- Kavak A, Yeşildal N, Parlak AH, Gökdemir G, Aydoğan I, Anul H, et al. Alopecia areata in Turkey: demographic and clinical features. J Eur Acad Dermatol Venereol. 2008;22:977–981. - PubMed
-
- Nanda A, Al-Fouzan AS, Al-Hasawi F. Alopecia areata in children: a clinical profile. Pediatr Dermatol. 2002;19:482–485. - PubMed
-
- Price VH. Alopecia areata: clinical aspects. J Invest Dermatol. 1991;96:68S. - PubMed
-
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. - PubMed
-
- Fiedler VC. Alopecia areata. A review of therapy, efficacy, safety, and mechanism. Arch Dermatol. 1992;128:1519–1529. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

