Surface Tension and Adsorption Studies by Drop Profile Analysis Tensiometry
- PMID: 29200810
- PMCID: PMC5686271
- DOI: 10.1007/s11743-017-2016-y
Surface Tension and Adsorption Studies by Drop Profile Analysis Tensiometry
Abstract
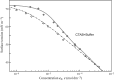
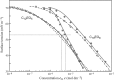
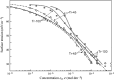
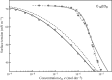
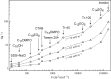
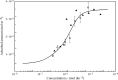
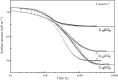
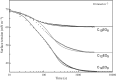
Surface tension and dilational viscoelasticity of solutions of various surfactants measured with bubble and drop profile analysis tensiometry are discussed. The study also includes experiments on the co-adsorption of surfactant molecules from a solution drop and alkane molecules from saturated alkane vapor phase. Using experimental data for 12 surfactants with different surface activities, it is shown that depletion due to adsorption of surfactant from the drop bulk can be significant. An algorithm is proposed quantitatively to take into consideration the depletion effect which is required for a correct description of the co-adsorption of alkanes on the solution drop surface and the correct analysis of experimental dynamic surface tension data to determine the adsorption mechanism. Bubble and drop profile analysis tensiometry is also the method of choice for measuring the dilational viscoelasticity of the adsorbed interfacial layer. The same elasticity moduli are obtained with the bubble and drop method only when the equilibrium surface pressures are sufficiently small (Π < 15 mN m-1). When the surface pressure for a surfactant solution is larger than this value, the viscoelasticity moduli determined from drop profile experiments become significantly larger than those obtained from bubble profile measurements.
Keywords: Bubble and drop profile analysis tensiometry; Surfactant adsorption layers; Surfactant depletion due to adsorption.
Figures







References
-
- Rosen MJ. The relationship of structure to properties in surfactants. J Am Oil Chem Soc. 1972;49:293–297. doi: 10.1007/BF02637577. - DOI
-
- Rosen MJ. The relationship of structure to properties in surfactants. Efficiency in surface or interfacial tension reduction. J Am Oil Chem Soc. 1974;51:461–465. doi: 10.1007/BF02635155. - DOI
-
- Abdel-Khalek NA, Omar AMA, Barakat Y. Relationship of structure to properties of some anionic surfactants as collectors in the flotation process. 1. Effect of chain length. J Chem Eng Data. 1999;44:133–137. doi: 10.1021/je9801359. - DOI
-
- Abdel-Khalek NA, Omar AMA. Relationship of structure to properties of some anionic surfactants as collectors in the flotation process. 2. Effect of phenyl group. J Chem Eng Data. 1999;44:138–141. doi: 10.1021/je980153q. - DOI
-
- Piispanen PS, Persson M, Claesson P, Norin T. Surface properties of surfactants derived from natural products. Part 1: syntheses and structure/property relationships—solubility and emulsification. J Surfact Deterg. 2004;7:147–159. doi: 10.1007/s11743-004-0298-6. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
