Notoginsenoside R1 attenuates high glucose-induced endothelial damage in rat retinal capillary endothelial cells by modulating the intracellular redox state
- PMID: 29200830
- PMCID: PMC5703151
- DOI: 10.2147/DDDT.S149700
Notoginsenoside R1 attenuates high glucose-induced endothelial damage in rat retinal capillary endothelial cells by modulating the intracellular redox state
Abstract
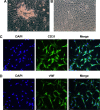
The aim of this study was to examine whether Notoginsenoside R1 (NR1) attenuates high glucose-induced cell damage in rat retinal capillary endothelial cells (RCECs) and to explore the mechanisms involved. The exposure of rat RCECs to high concentration of glucose (30 mM) for 72 h led to significant cytotoxicity, including decreased cell viability, reduced mitochondrial DNA copy number, increased lactate dehydrogenase release and elevated apoptosis. NR1, when present in the culture medium, markedly attenuated the high glucose-induced cytotoxicity in rat RCECs. Moreover, high glucose also induced a significant increase in intracellular reactive oxygen species and subsequently increased the activity of NADPH oxidase and poly-ADP (ribose) polymerase, whereas the activity of catalase decreased. The addition of NR1 to the medium significantly reduced the generation of reactive oxygen species, inhibited NADPH oxidase and poly-ADP (ribose) polymerase activities and increased catalase activity in RCECs, accompanied by a reduced cellular nitrotyrosine level. To explore the underlying mechanisms involved, the cellular redox status was monitored. Both the cellular NAD+ and NADPH levels decreased significantly in high glucose medium, which resulted in a marked decrease in the NAD+/NADH and NADPH/NADP+ ratios. High glucose stimulation also enhanced the accumulation of GSSG, maintaining the GSH/GSSG ratio lower than that in the control group with 5.5 mM glucose. When treated with NR1, the cellular NAD+, NADPH and GSH concentrations increased, and the ratios of NAD+/NADH, NADPH/NADP+ and GSH/GSSG increased, similar to the control group. These results demonstrate that NR1 attenuates high glucose-induced cell damage in RCECs. Therefore, NR1 may exert its protective effects via mechanisms that involve changes in the cellular redox state.
Keywords: NADPH; Notoginsenoside R1; apoptosis; high glucose; reactive oxygen species; retinal capillary endothelial cells.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



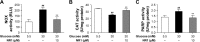

Similar articles
-
Ginsenoside Rb1 Attenuates High Glucose-Induced Oxidative Injury via the NAD-PARP-SIRT Axis in Rat Retinal Capillary Endothelial Cells.Int J Mol Sci. 2019 Oct 5;20(19):4936. doi: 10.3390/ijms20194936. Int J Mol Sci. 2019. PMID: 31590397 Free PMC article.
-
Astragaloside IV protects rat retinal capillary endothelial cells against high glucose-induced oxidative injury.Drug Des Devel Ther. 2017 Dec 13;11:3567-3577. doi: 10.2147/DDDT.S152489. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 29263652 Free PMC article.
-
Notoginsenoside R1 activates the NAMPT-NAD+-SIRT1 cascade to promote postischemic angiogenesis by modulating Notch signaling.Biomed Pharmacother. 2021 Aug;140:111693. doi: 10.1016/j.biopha.2021.111693. Epub 2021 May 21. Biomed Pharmacother. 2021. PMID: 34029951
-
Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death.Antioxid Redox Signal. 2005 Nov-Dec;7(11-12):1581-87. doi: 10.1089/ars.2005.7.1581. Antioxid Redox Signal. 2005. PMID: 16356121 Review.
-
Notoginsenoside R1: A systematic review of its pharmacological properties.Pharmazie. 2019 Nov 1;74(11):641-647. doi: 10.1691/ph.2019.9534. Pharmazie. 2019. PMID: 31739829
Cited by
-
Ginsenosides Rb1 and Rg1 Protect Primary Cultured Astrocytes against Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury via Improving Mitochondrial Function.Int J Mol Sci. 2019 Dec 3;20(23):6086. doi: 10.3390/ijms20236086. Int J Mol Sci. 2019. PMID: 31816825 Free PMC article.
-
Study on the mechanism of plant metabolites to intervene oxidative stress in diabetic retinopathy.Front Pharmacol. 2025 Feb 5;16:1517964. doi: 10.3389/fphar.2025.1517964. eCollection 2025. Front Pharmacol. 2025. PMID: 39974734 Free PMC article. Review.
-
Guan Xin Dan Shen formulation protects db/db mice against diabetic cardiomyopathy via activation of Nrf2 signaling.Mol Med Rep. 2021 Jul;24(1):531. doi: 10.3892/mmr.2021.12170. Epub 2021 May 26. Mol Med Rep. 2021. PMID: 34036388 Free PMC article.
-
Network Pharmacology-based Investigation of the Underlying Mechanism of Panax notoginseng Treatment of Diabetic Retinopathy.Comb Chem High Throughput Screen. 2020;23(4):334-344. doi: 10.2174/1386207323666200305093709. Comb Chem High Throughput Screen. 2020. PMID: 32133960 Free PMC article.
-
The Therapeutic Effect and Mechanism of Traditional Chinese Medicine in Type 2 Diabetes Mellitus and Its Complications.Diabetes Metab Syndr Obes. 2025 May 15;18:1599-1627. doi: 10.2147/DMSO.S517874. eCollection 2025. Diabetes Metab Syndr Obes. 2025. PMID: 40391051 Free PMC article. Review.
References
-
- Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res. 2005;37(Suppl 1):39–43. - PubMed
-
- Hosoya K, Tachikawa M. Inner blood-retinal barrier transporters: role of retinal drug delivery. Pharm Res. 2009;26(9):2055–2065. - PubMed
-
- Dagher Z, Park YS, Asnaghi V, et al. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Diabetes. 2004;53(9):2404–2411. - PubMed
-
- Williams KP, Steinle JJ. Maintenance of beta-adrenergic receptor signaling can reduce Fas signaling in human retinal endothelial cells. Exp Eye Res. 2009;89(4):448–455. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

