Solid lipid nanoparticles for thermoresponsive targeting: evidence from spectrophotometry, electrochemical, and cytotoxicity studies
- PMID: 29200845
- PMCID: PMC5701611
- DOI: 10.2147/IJN.S147506
Solid lipid nanoparticles for thermoresponsive targeting: evidence from spectrophotometry, electrochemical, and cytotoxicity studies
Erratum in
-
Erratum: Solid Lipid Nanoparticles for Thermoresponsive Targeting: Evidence from Spectrophotometry, Electrochemical, and Cytotoxicity Studies [Corrigendum].Int J Nanomedicine. 2021 Jul 30;16:5187. doi: 10.2147/IJN.S330459. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34354352 Free PMC article.
Abstract
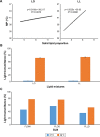
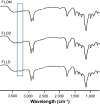
Thermoresponsive drug delivery systems are designed for the controlled and targeted release of therapeutic payload. These systems exploit hyperthermic temperatures (>39°C), which may be applied by some external means or due to an encountered symptom in inflammatory diseases such as cancer and arthritis. The objective of this paper was to provide some solid evidence in support of the hypothesis that solid lipid nanoparticles (SLNs) can be used for thermoresponsive targeting by undergoing solid-liquid phase transition at their melting point (MP). Thermoresponsive lipid mixtures were prepared by mixing solid and liquid natural fatty acids, and their MP was measured by differential scanning calorimetry (DSC). SLNs (MP 39°C) containing 5-fluorouracil (5-FU) were synthesized by hot melt encapsulation method, and were found to have spherical shape (transmission electron microscopy studies), desirable size (<200 nm), and enhanced physicochemical stability (Fourier transform infrared spectroscopy analysis). We observed a sustained release pattern (22%-34%) at 37°C (5 hours). On the other hand, >90% drug was released at 39°C after 5 hours, suggesting that the SLNs show thermoresponsive drug release, thus confirming our hypothesis. Drug release from SLNs at 39°C was similar to oleic acid and linoleic acid nanoemulsions used in this study, which further confirmed that thermoresponsive drug release is due to solid-liquid phase transition. Next, a differential pulse voltammetry-based electrochemical chemical detection method was developed for quick and real-time analysis of 5-FU release, which also confirmed thermoresponsive drug release behavior of SLNs. Blank SLNs were found to be biocompatible with human gingival fibroblast cells, although 5-FU-loaded SLNs showed some cytotoxicity after 24 hours. 5-FU-loaded SLNs showed thermoresponsive cytotoxicity to breast cancer cells (MDA-MB-231) as cytotoxicity was higher at 39°C (cell viability 72%-78%) compared to 37°C (cell viability >90%) within 1 hour. In conclusion, this study presents SLNs as a safe, simple, and effective platform for thermoresponsive targeting.
Keywords: 5-fluorouracil; breast cancer; emulsions; fatty acids; nanostructured lipid carriers; temperature sensitive.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





References
-
- Dou Y, Hynynen K, Allen C. To heat or not to heat: challenges with clinical translation of thermosensitive liposomes. J Control Release. 2017;249:63–73. - PubMed
-
- Madni A, Sarfraz M, Rehman M, et al. Liposomal drug delivery: a versatile platform for challenging clinical applications. J Pharm Pharm Sci. 2014;17(3):401–426. - PubMed
-
- Lu T, ten Hagen TL. Inhomogeneous crystal grain formation in DPPC-DSPC based thermosensitive liposomes determines content release kinetics. J Control Release. 2016;247:64–72. - PubMed
-
- Al-Ahmady Z, Kostarelos K. Chemical components for the design of temperature-responsive vesicles as cancer therapeutics. Chem Rev. 2016;116(6):3883–3918. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

