Cost Utility Analysis of Cervical Therapeutic Medial Branch Blocks in Managing Chronic Neck Pain
- PMID: 29200944
- PMCID: PMC5707747
- DOI: 10.7150/ijms.20755
Cost Utility Analysis of Cervical Therapeutic Medial Branch Blocks in Managing Chronic Neck Pain
Abstract
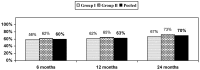
Background: Controlled diagnostic studies have established the prevalence of cervical facet joint pain to range from 36% to 67% based on the criterion standard of ≥ 80% pain relief. Treatment of cervical facet joint pain has been described with Level II evidence of effectiveness for therapeutic facet joint nerve blocks and radiofrequency neurotomy and with no significant evidence for intraarticular injections. However, there have not been any cost effectiveness or cost utility analysis studies performed in managing chronic neck pain with or without headaches with cervical facet joint interventions. Study Design: Cost utility analysis based on the results of a double-blind, randomized, controlled trial of cervical therapeutic medial branch blocks in managing chronic neck pain. Objectives: To assess cost utility of therapeutic cervical medial branch blocks in managing chronic neck pain. Methods: A randomized trial was conducted in a specialty referral private practice interventional pain management center in the United States. This trial assessed the clinical effectiveness of therapeutic cervical medial branch blocks with or without steroids for an established diagnosis of cervical facet joint pain by means of controlled diagnostic blocks. Cost utility analysis was performed with direct payment data for the procedures for a total of 120 patients over a period of 2 years from this trial based on reimbursement rates of 2016. The payment data provided direct procedural costs without inclusion of drug treatments. An additional 40% was added to procedural costs with multiplication of a factor of 1.67 to provide estimated total costs including direct and indirect costs, based on highly regarded surgical literature. Outcome measures included significant improvement defined as at least a 50% improvement with reduction in pain and disability status with a combined 50% or more reduction in pain in Neck Disability Index (NDI) scores. Results: The results showed direct procedural costs per one-year improvement in quality adjusted life year (QALY) of United States Dollar (USD) of $2,552, and overall costs of USD $4,261. Overall, each patient on average received 5.7 ± 2.2 procedures over a period of 2 years. Average significant improvement per procedure was 15.6 ± 12.3 weeks and average significant improvement in 2 years per patient was 86.0 ± 24.6 weeks. Limitations: The limitations of this cost utility analysis are that data are based on a single center evaluation. Only costs of therapeutic interventional procedures and physician visits were included, with extrapolation of indirect costs. Conclusion: The cost utility analysis of therapeutic cervical medial branch blocks in the treatment of chronic neck pain non-responsive to conservative management demonstrated clinical effectiveness and cost utility at USD $4,261 per one year of QALY.
Keywords: Chronic neck pain; cervical facet joint pain; cervical medial branch blocks; controlled diagnostic blocks; cost effectiveness analysis; cost utility analysis; quality adjusted life years (QALY)..
Conflict of interest statement
Competing Interests: Dr. Manchikanti has provided limited consulting services to Semnur Pharmaceuticals, Incorporated, which is developing nonparticulate steroids. Dr. Kaye is a speaker for Depomed and Merck, Inc. Dr. Hirsch is a consultant for Medtronic.
Figures

Similar articles
-
Comparative outcomes of a 2-year follow-up of cervical medial branch blocks in management of chronic neck pain: a randomized, double-blind controlled trial.Pain Physician. 2010 Sep-Oct;13(5):437-50. Pain Physician. 2010. PMID: 20859313 Clinical Trial.
-
Outcomes of Cervical Therapeutic Medial Branch Blocks and Radiofrequency Neurotomy: Clinical Outcomes and Cost Utility are Equivalent.Pain Physician. 2022 Jan;25(1):35-47. Pain Physician. 2022. PMID: 35051143
-
Cervical Interlaminar Epidural Injections in the Treatment of Cervical Disc Herniation, Post Surgery Syndrome, or Discogenic Pain: Cost Utility Analysis from Randomized Trials.Pain Physician. 2019 Sep;22(5):421-431. Pain Physician. 2019. PMID: 31561644
-
Cervical zygapophysial (facet) joint pain: effectiveness of interventional management strategies.Postgrad Med. 2016 Jan;128(1):54-68. doi: 10.1080/00325481.2016.1105092. Epub 2015 Dec 10. Postgrad Med. 2016. PMID: 26653406 Review.
-
Effectiveness of Facet Joint Nerve Blocks in Managing Chronic Axial Spinal Pain of Facet Joint Origin: A Systematic Review and Meta-Analysis.Pain Physician. 2024 Feb;27(2):E169-E206. Pain Physician. 2024. PMID: 38324785
Cited by
-
Utilization of Vertebral Augmentation Procedures in the USA: a Comparative Analysis in Medicare Fee-for-Service Population Pre- and Post-2009 Trials.Curr Pain Headache Rep. 2020 Apr 14;24(5):22. doi: 10.1007/s11916-020-00850-2. Curr Pain Headache Rep. 2020. PMID: 32291587 Review.
-
Treatment of Discogenic Low Back Pain: Current Treatment Strategies and Future Options-a Literature Review.Curr Pain Headache Rep. 2019 Nov 9;23(11):86. doi: 10.1007/s11916-019-0821-x. Curr Pain Headache Rep. 2019. PMID: 31707499 Review.
-
Comparison of effectiveness for fluoroscopic cervical interlaminar epidural injections with or without steroid in cervical post-surgery syndrome.Korean J Pain. 2018 Oct;31(4):277-288. doi: 10.3344/kjp.2018.31.4.277. Epub 2018 Oct 1. Korean J Pain. 2018. PMID: 30310553 Free PMC article.
-
Regenerative Injection Treatments Utilizing Platelet Products and Prolotherapy for Cervical Spine Pain: A Functional Spinal Unit Approach.Cureus. 2021 Oct 8;13(10):e18608. doi: 10.7759/cureus.18608. eCollection 2021 Oct. Cureus. 2021. PMID: 34659923 Free PMC article.
-
Evaluation of Cost-Utility of Thoracic Interlaminar Epidural Injections.Curr Pain Headache Rep. 2020 Jan 30;24(3):5. doi: 10.1007/s11916-020-0838-1. Curr Pain Headache Rep. 2020. PMID: 32002687
References
-
- Manchikanti L, Singh V, Datta S. et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35–70. - PubMed
-
- Côté P, Cassidy JD, Carroll L. The Saskatchewan Health and Back Pain Survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine (Phila Pa 1976) 1998;23:1689–98. - PubMed
-
- Hoy D, March L, Woolf A. et al. The global burden of neck pain: Estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1309–15. - PubMed
-
- Martin BI, Deyo RA, Mirza SK. et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

