High Concentrations of Rosiglitazone Reduce mRNA and Protein Levels of LRP1 in HepG2 Cells
- PMID: 29201005
- PMCID: PMC5696635
- DOI: 10.3389/fphar.2017.00772
High Concentrations of Rosiglitazone Reduce mRNA and Protein Levels of LRP1 in HepG2 Cells
Abstract
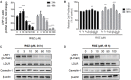
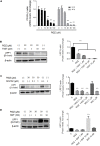
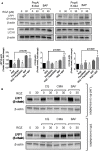
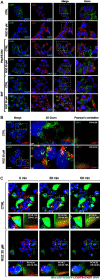
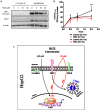
Low-density lipoprotein receptor-related protein 1 (LRP1) is an endocytic receptor involved in the uptake of a variety of molecules, such as apoE, α2-macroglobulin, and the amyloid β peptide (Aβ), for either transcellular transport, protein trafficking or lysosomal degradation. The LRP1 gene can be transcribed upon activation of peroxisome proliferator receptor activated-γ (PPARγ) by the potent PPARγ agonist, rosiglitazone (RGZ). In previous studies, RGZ was shown to upregulate LRP1 levels in concentrations between 0.1 and 5 μM in HepG2 cells. In this study, we sought to replicate previous studies and to investigate the molecular mechanism by which high concentrations of RGZ reduce LRP1 levels in HepG2 cells. Our data confirmed that transcriptional activation of LRP1 occurred in response to RGZ at 3 and 10 μM, in agreement with the study reported by Moon et al. (2012a). On the other hand, we found that high concentrations of RGZ decreased both mRNA and protein levels of LRP1. Mechanistically, transcriptional dysregulation of LRP1 was affected by the downregulation of PPARγ in a time- and concentration-dependent manner. However, downregulation of PPARγ was responsible for only 40% of the LRP1 reduction and thereby the remaining loss of LRP1 (60%) was found to be through degradation in the lysosomal system. In conclusion, our findings demonstrate the mechanisms by which high concentrations of RGZ caused LRP1 levels to be reduced in HepG2 cells. Taken together, this data will be helpful to better explain the pharmacological modulation of this pivotal membrane receptor by PPARγ agonists.
Keywords: LRP1; PPARγ; bafilomycin A1; lysosomal degradation; protein degradation; rosiglitazone.
Figures
References
-
- Cal R., García-Arguinzonis M., Revuelta-López E., Castellano J., Padró T., Badimon L., et al. (2013). Aggregated low-density lipoprotein induces LRP1 stabilization through E3 ubiquitin ligase CHFR downregulation in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 33 369–377. 10.1161/ATVBAHA.112.300748 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

