PifC and Osa, Plasmid Weapons against Rival Conjugative Coupling Proteins
- PMID: 29201021
- PMCID: PMC5696584
- DOI: 10.3389/fmicb.2017.02260
PifC and Osa, Plasmid Weapons against Rival Conjugative Coupling Proteins
Abstract
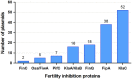
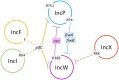
Bacteria display a variety of mechanisms to control plasmid conjugation. Among them, fertility inhibition (FI) systems prevent conjugation of co-resident plasmids within donor cells. Analysis of the mechanisms of inhibition between conjugative plasmids could provide new alternatives to fight antibiotic resistance dissemination. In this work, inhibition of conjugation of broad host range IncW plasmids was analyzed in the presence of a set of co-resident plasmids. Strong FI systems against plasmid R388 conjugation were found in IncF/MOBF12 as well as in IncI/MOBP12 plasmids, represented by plasmids F and R64, respectively. In both cases, the responsible gene was pifC, known also to be involved in FI of IncP plasmids and Agrobacterium T-DNA transfer to plant cells. It was also discovered that the R388 gene osa, which affects T-DNA transfer, also prevented conjugation of IncP-1/MOBP11 plasmids represented by plasmids RP4 and R751. Conjugation experiments of different mobilizable plasmids, helped by either FI-susceptible or FI-resistant transfer systems, demonstrated that the conjugative component affected by both PifC and Osa was the type IV conjugative coupling protein. In addition, in silico analysis of FI proteins suggests that they represent recent acquisitions of conjugative plasmids, i.e., are not shared by members of the same plasmid species. This implies that FI are rapidly-moving accessory genes, possibly acting on evolutionary fights between plasmids for the colonization of specific hosts.
Keywords: Osa protein; PifC protein; antimicrobial resistance; bacterial conjugation; fertility inhibition; mobilization; plasmid; type IV coupling protein.
Figures




Similar articles
-
F factor inhibition of conjugal transfer of broad-host-range plasmid RP4: requirement for the protein product of pif operon regulatory gene pifC.J Bacteriol. 1985 Sep;163(3):1067-73. doi: 10.1128/jb.163.3.1067-1073.1985. J Bacteriol. 1985. PMID: 2993231 Free PMC article.
-
Plasmid pB8 is closely related to the prototype IncP-1beta plasmid R751 but transfers poorly to Escherichia coli and carries a new transposon encoding a small multidrug resistance efflux protein.Plasmid. 2005 Sep;54(2):135-48. doi: 10.1016/j.plasmid.2005.03.001. Epub 2005 Apr 18. Plasmid. 2005. PMID: 16122561
-
Genetic organization of the conjugal DNA processing region of the IncW plasmid R388.J Mol Biol. 1994 Jan 14;235(2):448-64. doi: 10.1006/jmbi.1994.1005. J Mol Biol. 1994. PMID: 8289274
-
Natural and Artificial Strategies To Control the Conjugative Transmission of Plasmids.Microbiol Spectr. 2018 Jan;6(1):10.1128/microbiolspec.mtbp-0015-2016. doi: 10.1128/microbiolspec.MTBP-0015-2016. Microbiol Spectr. 2018. PMID: 29327679 Free PMC article. Review.
-
Towards a better understanding of antimicrobial resistance dissemination: what can be learnt from studying model conjugative plasmids?Mil Med Res. 2022 Jan 10;9(1):3. doi: 10.1186/s40779-021-00362-z. Mil Med Res. 2022. PMID: 35012680 Free PMC article. Review.
Cited by
-
Dominance Between Plasmids Determines the Extent of Biofilm Formation.Front Microbiol. 2020 Aug 26;11:2070. doi: 10.3389/fmicb.2020.02070. eCollection 2020. Front Microbiol. 2020. PMID: 32983050 Free PMC article.
-
Cis-Acting Relaxases Guarantee Independent Mobilization of MOBQ 4 Plasmids.Front Microbiol. 2019 Nov 8;10:2557. doi: 10.3389/fmicb.2019.02557. eCollection 2019. Front Microbiol. 2019. PMID: 31781067 Free PMC article.
-
Plasmid profiling and incompatibility grouping of multidrug resistant Salmonella enterica serovar Typhi isolates in Nairobi, Kenya.BMC Res Notes. 2019 Jul 16;12(1):422. doi: 10.1186/s13104-019-4468-9. BMC Res Notes. 2019. PMID: 31311578 Free PMC article.
-
Conjugation's Toolkit: the Roles of Nonstructural Proteins in Bacterial Sex.J Bacteriol. 2023 Mar 21;205(3):e0043822. doi: 10.1128/jb.00438-22. Epub 2023 Feb 27. J Bacteriol. 2023. PMID: 36847532 Free PMC article. Review.
-
Targeted bacterial conjugation mediated by synthetic cell-to-cell adhesions.Nucleic Acids Res. 2022 Dec 9;50(22):12938-12950. doi: 10.1093/nar/gkac1164. Nucleic Acids Res. 2022. PMID: 36511856 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

