Comparative Analysis of Spontaneous and Stimulus-Evoked Calcium Transients in Proliferating and Differentiating Human Midbrain-Derived Stem Cells
- PMID: 29201062
- PMCID: PMC5671755
- DOI: 10.1155/2017/9605432
Comparative Analysis of Spontaneous and Stimulus-Evoked Calcium Transients in Proliferating and Differentiating Human Midbrain-Derived Stem Cells
Abstract
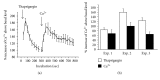
Spontaneous cytosolic calcium transients and oscillations have been reported in various tissues of nonhuman and human origin but not in human midbrain-derived stem cells. Using confocal microfluorimetry, we studied spontaneous calcium transients and calcium-regulating mechanisms in a human ventral mesencephalic stem cell line undergoing proliferation and neuronal differentiation. Spontaneous calcium transients were detected in a large fraction of both proliferating (>50%) and differentiating (>55%) cells. We provide evidence for the existence of intracellular calcium stores that respond to muscarinic activation of the cells, having sensitivity for ryanodine and thapsigargin possibly reflecting IP3 receptor activity and the presence of ryanodine receptors and calcium ATPase pumps. The observed calcium transient activity potentially supports the existence of a sodium-calcium antiporter and the existence of calcium influx induced by depletion of calcium stores. We conclude that the cells have developed the most important mechanisms governing cytosolic calcium homeostasis. This is the first comparative report of spontaneous calcium transients in proliferating and differentiating human midbrain-derived stem cells that provides evidence for the mechanisms that are likely to be involved. We propose that the observed spontaneous calcium transients may contribute to mechanisms involved in cell proliferation, phenotypic differentiation, and general cell maturation.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

