Inhibition of penile tunica albuginea myofibroblasts activity by adipose-derived stem cells
- PMID: 29201230
- PMCID: PMC5704342
- DOI: 10.3892/etm.2017.5179
Inhibition of penile tunica albuginea myofibroblasts activity by adipose-derived stem cells
Abstract
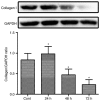
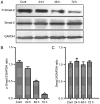
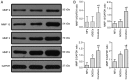
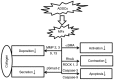
The activation of tunica albuginea myofibroblasts (MFs) serves an essential role in Peyronie's disease (PD). Increasing evidence has reported that adipose tissue-derived stem cells (ADSCs) have been demonstrated to attenuate the symptoms of PD in animal models. However, the mechanisms of the antifibrotic effects of ADSCs in PD remain to be fully elucidated. In the present study, the inhibitory effects and possible mechanism of ADSCs on the activation of MFs derived from rat penile tunica albuginea were investigated. ADSCs were obtained from the paratesticular fat of Sprague Dawley rats. MFs were transformed from rat penile tunica albuginea fibroblasts through stimulation with 5 ng/ml tumor growth factor-β1. Transwell cell cultures were adopted for co-culture of ADSCs and MFs. Western blot analysis was used to assess changes in the expression levels of α smooth muscle actin (αSMA), collagen I, phosphorylated (p)-SMAD family member 2 (Smad2), Smad2, ras homolog family member A (RhoA), Rho associated coiled-coil containing protein kinase (ROCK)1 and ROCK2, caspase3, caspase9, and matrix metalloproteinases (MMPs). Collagen gel assays were used to assess cell contractility. Additionally, the concentration of hydroxyproline in the culture medium was detected using commercially available kits. It was demonstrated that ADSCs reduced the expression of αSMA and collagen I of MFs. Furthermore, p-Smad2, RhoA, ROCK1 and ROCK2 expression was significantly reduced in the MFs+ADSCs group compared with that in the MFs-only culture, while the expression of MMPs (MMP2, MMP3, MMP9 and MMP13) and caspases (caspase3 and caspase9) was upregulated. In addition, ADSCs were able to downregulate the concentration of hydroxyproline in the culture medium of MFs and reverse the contraction of MFs. Collectively, these results suggested that ADSCs inhibited the activation of MFs, decreased collagen production, and suppressed the contraction of myofibroblasts, via Smad and RhoA/ROCK signaling pathways. Furthermore, ADSCs reduced the deposition of collagen and promoted the apoptosis of MFs via MMPs, and caspases. Accordingly, the application of ADSCs may provide a novel therapeutic strategy for PD.
Keywords: Peyronie's disease; adipose tissue-derived stem cells; fibrosis; myofibroblasts.
Figures



References
-
- Jiang HS, Zhu LL, Zhang Z, Chen H, Chen Y, Dai YT. Estradiol attenuates the TGF-β1-induced conversion of primary TAFs into myofibroblasts and inhibits collagen production and myofibroblast contraction by modulating the Smad and Rho/ROCK signaling pathways. Int J Mol Med. 2015;36:801–807. doi: 10.3892/ijmm.2015.2288. - DOI - PubMed
-
- Vernet D, Ferrini MG, Valente EG, Magee TR, Bou-Gharios G, Rajfer J, Gonzalez-Cadavid NF. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie's fibrotic plaque and in its rat model. Nitric Oxide. 2002;7:262–276. doi: 10.1016/S1089-8603(02)00124-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
