Use of wearable devices for post-discharge monitoring of ICU patients: a feasibility study
- PMID: 29201377
- PMCID: PMC5698959
- DOI: 10.1186/s40560-017-0261-9
Use of wearable devices for post-discharge monitoring of ICU patients: a feasibility study
Abstract
Background: Wearable devices generate signals detecting activity, sleep, and heart rate, all of which could enable detailed and near-continuous characterization of recovery following critical illness.
Methods: To determine the feasibility of using a wrist-worn personal fitness tracker among patients recovering from critical illness, we conducted a prospective observational study of a convenience sample of 50 stable ICU patients. We assessed device wearability, the extent of data capture, sensitivity and specificity for detecting heart rate excursions, and correlations with questionnaire-derived sleep quality measures.
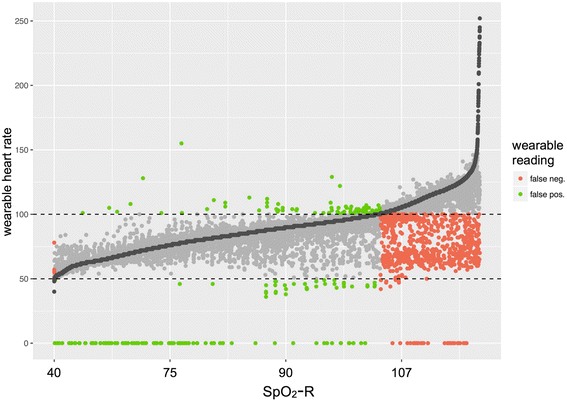
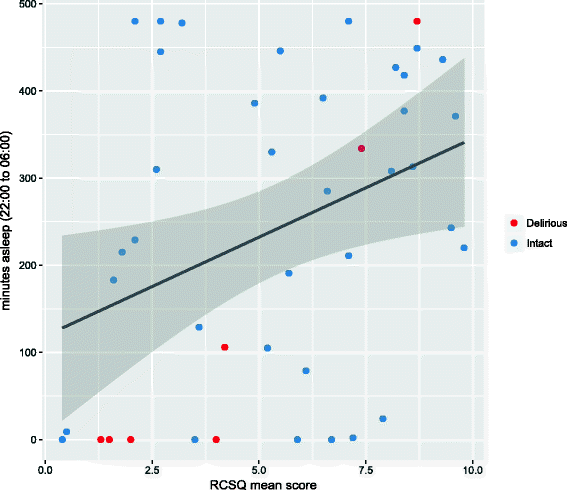
Results: Wearable devices were worn over a 24-h period, with excellent capture of data. While specificity for the detection of tachycardia was high (98.8%), sensitivity was low to moderate (69.5%). There was a moderate correlation between wearable-derived sleep duration and questionnaire-derived sleep quality (r = 0.33, P = 0.03). Devices were well-tolerated and demonstrated no degradation in quality of data acquisition over time.
Conclusions: We found that wearable devices could be worn by patients recovering from critical illness and could generate useful data for the majority of patients with little adverse effect. Further development and study are needed to better define and enhance the role of wearables in the monitoring of post-ICU recovery.
Trial registration: Clinicaltrials.gov, NCT02527408.
Keywords: Critical care; Heart rate monitoring; Medical informatics; Mobile health technologies; Sleep quality; Validation study; Wearable devices.
Conflict of interest statement
Ethics approval and consent to participate
All participating patients, or substitute decision makers on their behalf, provided written informed consent for participation in this study. The Health Sciences Research Ethics Board at Queen’s University reviewed and approved the study protocol, and the trial was registered with
Consent for publication
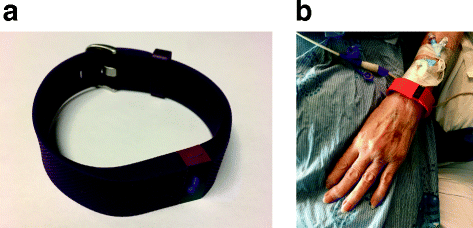
The patient depicted in Fig. 1 provided consent for the photo to be published in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Salah H, MacIntosh E, Rajakulendran N. Wearable tech: leveraging canadian innovation to improve health: MaRS Market Insights; 2014 Mar 26. p. 1–45. Available from https://www.marsdd.com/wp-content/uploads/2015/02/MaRSReport-WearableTec...
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

