Identifying the hidden burden of allergic rhinitis (AR) in community pharmacy: a global phenomenon
- PMID: 29201385
- PMCID: PMC5696909
- DOI: 10.1186/s40733-017-0036-z
Identifying the hidden burden of allergic rhinitis (AR) in community pharmacy: a global phenomenon
Abstract
Background: Patients with allergic rhinitis often trivialise their condition, self-manage inappropriately, and would benefit from health care intervention. The primary point of health care contact for these self-managing allergic rhinitis patients is the community pharmacy. With the majority of allergic rhinitis treatments being available for purchase over the counter, without health care professional contact, we know little about how the patients self-manage. This study aims to identify the burden of allergic rhinitis in the community pharmacy and to identify key opportunity for intervention.
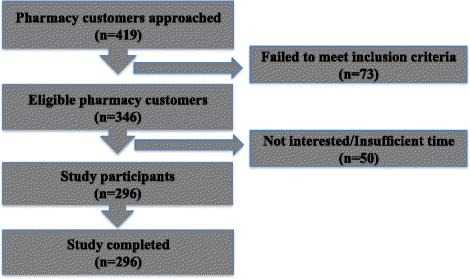
Methods: Pharmacy customers, who purchased nasal treatment in a community pharmacy, were approached with a research-administered questionnaire that collected data on medical history, symptoms and products purchased for the treatment of nasal symptoms.
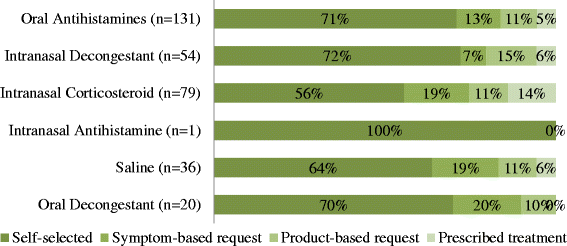
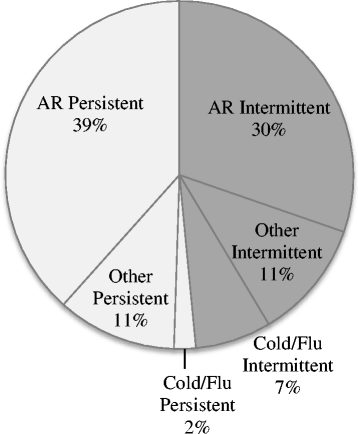
Results: Of the 296 participants, 69.9% self-managed with over-the-counter medications; with 68% experiencing allergic rhinitis symptoms and only 44.3% of this subgroup had a doctor's diagnosis. Nasal congestion (73.6%) was most commonly experienced and oral antihistamines were most commonly purchased (44.3%), indicating a pattern of suboptimal management. A third of participants (36.5%) experienced moderate-severe symptoms, persistently, which impacted on their daily living. Medication selection was mainly based on pharmacy customers' perceptions of medication effectiveness (47.6%).
Conclusion: A majority of participants that self-selected over-the-counter medications have symptoms consistent with allergic rhinitis, with almost half not having received a diagnosis. Medication purchasing patterns suggest that sub-optimal therapeutic decisions made by participants, even when they are experiencing significant symptoms. This study uncovers the hidden burden of allergic rhinitis in the community pharmacy and a missed opportunity to intervene and refer if necessary. Patients need to be guided through appropriate treatment as this study showed that many should be referred to a medical practitioner.
Keywords: Allergic rhinitis; Consult pharmacists; Self-manage; Suboptimal management.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained by the University of Sydney Human Research Ethics Committee (Reference No. 2015/527).
Consent for publication
Not applicable
Competing interests
The authors involved in this research are Rachel Tan, Biljana Cvetkovski, Vicky Kritikos, David Price, Kwok Yan, Pete Smith and Sinthia Bosnic-Anticevich. Rachel Tan and Biljana Cvetkovski declare that they have no competing interest. Vicky Kritikos has received honoraria from AstraZeneca, GlaxoSmithKline and Pfizer. Kwok Yan has received honoraria for speaking and consulting from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Meda, Mundipharma and Pfizer. Pete Smith is a clinical allergist with research and clinical interest in rhinology and has also been a speaker for Meda, GlaxoSmithKline, Novartis, Mundipharma and AstraZeneca. David Price has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from UK National Health Service, British Lung Foundation, Aerocrine, AKL Research and Development Ltd., AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Takeda, Teva Pharmaceuticals, Zentiva, and Theravance; payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Novartis and Mundipharma; payment for travel/accommodation/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, Teva Pharmaceuticals, and AstraZeneca; funding for patient enrolment or completion of research from Chiesi, Teva Pharmaceuticals, Zentiva, and Novartis; stock/stock options from AKL Research and Development Ltd., which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd., UK, and 74% of Observational and Pragmatic Research Institute Pte Ltd., Singapore; and is peer reviewer for grant committees of the Medical Research Council, Efficacy and Mechanism Evaluation programme, and Health Technology Assessment. Sinthia Bosnic-Anticevich is a member of the Teva Pharmaceuticals Devices International Key Experts Panel, has received research support from Research in Real Life, has received lecture fees and payment for developing educational presentations from Teva and Mundipharma; and has received Honoria from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, for her contribution to advisory boards/key international expert forum.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. - DOI - PubMed
-
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P et al: European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012(23):3 p preceding table of contents, 1–298. - PubMed
-
- Pawankar R, Canonica R, Holgate S, Lockey R, Blaiss M. World allergy organisation (WAO) white book on allergy: update 2013. Milwaukee: World Allergy Organization; 2013.
-
- Pawankar R, Canonica G, Holgate S, Loceky R. World Allergy Organisation (WAO): White book on allergy. Wisconsin: World Allergy Organisation; 2011. Available online at: http://www.worldallergy.org/UserFiles/file/WAO-White-Book-on-Allergy_web....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

