Evaluating the feasibility of conducting a trial using a patient decision aid in implantable cardioverter defibrillator candidates: a randomized controlled feasibility trial
- PMID: 29201388
- PMCID: PMC5697082
- DOI: 10.1186/s40814-017-0189-9
Evaluating the feasibility of conducting a trial using a patient decision aid in implantable cardioverter defibrillator candidates: a randomized controlled feasibility trial
Abstract
Background: Patient decision aids (PtDA) support quality decision-making. The aim of this research was to evaluate the feasibility of conducting a randomized controlled trial delivering an implantable cardioverter defibrillator (ICD)-specific PtDA to new ICD candidates and examining preliminary estimates of differences in outcomes.
Methods: Prior to recruitment, ICD candidacy was determined. Consented patients were randomized to (1) usual care or (2) PtDA intervention. Feasibility outcomes included referral and recruitment rates, successful PtDA delivery, and completion of measures. The PtDA intervention was administered prior to specialist consultation and baseline demographics, and measures of decision quality including decisional conflict (DCS), SURE test (Sure of myself, Understand information, Risk-benefit ratio, Encouragement), patient's ICD specific values, ICD knowledge, and health-related quality of life were recorded. Post-consultation, participant's DCS was repeated and decisions to proceed, decline, or defer ICD implantation were collected. Feasibility data was determined using descriptive statistics (continuous and categorical). Preliminary estimates of differences in outcomes were assessed using mean differences. Concordance between values and decision choice was assessed using logistic regression of the intervention group.
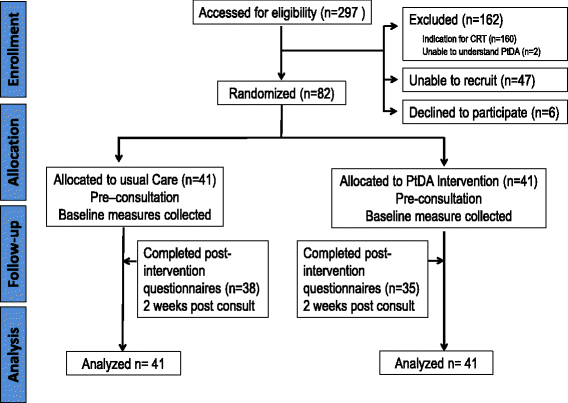
Results: We identified 135 eligible patients. Eighty-two consented to the trial randomizing patients to usual care (n = 41) or PtDA intervention (n = 41). Feasibility outcome results were (1) referral rate at approximately 20/month, (2) recruitment rate 61%, and (3) successful delivery of PtDA and study management. Pre-consultation, PtDA patients scored lower on the DCS scale (mean, standard deviation [SD] 27.3 (18.4) compared to usual care, 49.4 (18.6); the between-group difference in means [95% confidence interval (CI)] was - 22.1[- 30.23, - 13.97]. A difference remained post-implantation 21.2 (11.7), PtDA intervention 29.9 (13.3), and usual care - 8.7 [- 14.61, - 2.86]. SURE test results supported DCS differences. The PtDA group scored higher on the ICD-related knowledge questions, with 47.50% scoring greater than 3/5 of the knowledge questions correct, compared to 23.09% receiving usual care. The mean [SD] number of correct knowledge responses out of 5 was 3.33(1.19) in the PtDA group and 2.62 (1.16) in usual care pre-implant. Concordance between values and decision choice found a strong association between predicted and actual ICD implant status in the intervention group.
Conclusion: Our results suggest that a future definitive trial is feasible. The ICD-specific PtDA shows promise with respect to preliminary estimates of differences in outcomes.
Trial registration: NCT01876173.
Keywords: Decision aid; Implantable cardio-defibrillator; Knowledge translation; Shared decision-making.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Hamilton Integrated Research Ethics Board (HIREB 12-214).
Informed consent was obtained from all study participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Tang AS, Ross H, Simpson CS, Mitchell LB, Dorian P, Goeree R, Hoffmaster B, Arnold M, Talajic M, on behalf of the Canadian Heart Rhythm S and the Canadian Cardiovascular S Canadian Cardiovascular Society/Canadian Heart Rhythm Society position paper on implantable cardioverter defibrillator use in Canada. Can J Cardiol. 2005;21:11A–18A. - PubMed
-
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, American Heart Association Statistics C and Stroke Statistics S Heart disease and stroke statistics—2010 update: a report from the American Heart Association. [Erratum appears in Circulation. 2010 Mar 30;121(12):e260 Note: Stafford, Randall [corrected to Roger, Veronique L]] Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. - DOI - PubMed
-
- Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada P, Camm AJ, Cappato R, Cobbe SM, Di Mario C, Maron BJ, McKenna WJ, Pedersen AK, Ravens U, Schwartz PJ, Trusz-Gluza M, Vardas P, Wellens HJJ, Zipes DP. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J. 2001;22:1374–1450. doi: 10.1053/euhj.2001.2824. - DOI - PubMed
-
- Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, Greene HL, Boczor S, Domanski M, Follmann D, Gent M, Roberts RS. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–2078. doi: 10.1053/euhj.2000.2476. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical