In Situ Ligation of High- and Low-Affinity Ligands to Cell Surface Receptors Enables Highly Selective Recognition
- PMID: 29201607
- PMCID: PMC5700463
- DOI: 10.1002/advs.201700147
In Situ Ligation of High- and Low-Affinity Ligands to Cell Surface Receptors Enables Highly Selective Recognition
Abstract
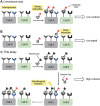
This paper reports an entirely unexplored concept of simultaneously recognizing two receptors using high- and low-affinity ligands through ligating them in situ on the target cell surface. This de novo approach is inspired by the pretargeting strategy frequently applied in molecular imaging, and has now evolved as the basis of a new paradigm for visualizing target cells with a high imaging contrast. A distinct advantage of using a labeled low-affinity ligand such as glycan is that the excess labeled ligand can be washed away from the cells, whereas the ligand bound to the cell, even at the milli molar affinity level, can be anchored by a bioorthogonal reaction with a pretargeted high-affinity ligand on the surface. Consequently, nonspecific background is minimized, leading to improved imaging contrast. Importantly, despite previously unexplored for molecular imaging, a notoriously weak glycan/lectin interaction can now be utilized as a highly selective ligand to the targets.
Keywords: cell imaging; cell surfaces; glycan; in situ ligation.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
