Immunization with Bivalent Flagellin Protects Mice against Fatal Pseudomonas aeruginosa Pneumonia
- PMID: 29201922
- PMCID: PMC5671732
- DOI: 10.1155/2017/5689709
Immunization with Bivalent Flagellin Protects Mice against Fatal Pseudomonas aeruginosa Pneumonia
Abstract
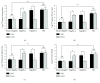
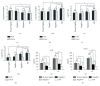
Pseudomonas aeruginosa lung infections present a major challenge to healthcare systems worldwide because they are commonly associated with high morbidity and mortality. Here, we demonstrate the protective efficacy of type a and b flagellins (bivalent flagellin) against acute fatal pneumonia in mice. Mice immunized intranasally with a bivalent flagellin vaccine were challenged by different flagellated strains of P. aeruginosa in an acute pneumonia model. Besides the protective effect of the vaccine, we further measured the host innate and cellular immunity responses. The immunized mice in our study were protected against both strains. Remarkably, active immunization with type a or b flagellin significantly improved survival of mice against heterologous strain compared to flagellin a or b antisera. We also showed that after an intranasal challenge by P. aeruginosa strain, neutrophils are recruited to the airways of vaccinated mice, and that the bivalent flagellin vaccine was proved to be protective by the generated CD4+IL-17+ Th17 cells. In conclusion, bivalent flagellin vaccine can confer protection against different strains of P. aeruginosa in an acute pneumonia mouse model by eliciting effective cellular and humoral immune responses, including increased IL-17 production and improved opsonophagocytic killing.
Figures








Similar articles
-
Immunization with outer membrane proteins (OprF and OprI) and flagellin B protects mice from pulmonary infection with mucoid and nonmucoid Pseudomonas aeruginosa.J Microbiol Immunol Infect. 2018 Jun;51(3):312-320. doi: 10.1016/j.jmii.2016.08.014. Epub 2017 Feb 20. J Microbiol Immunol Infect. 2018. PMID: 28291719
-
Bivalent flagellin immunotherapy protects mice against Pseudomonas aeruginosa infections in both acute pneumonia and burn wound models.Biologicals. 2017 Mar;46:29-37. doi: 10.1016/j.biologicals.2016.12.005. Epub 2017 Jan 6. Biologicals. 2017. PMID: 28065582
-
Protective efficacy of Pseudomonas aeruginosa type-A flagellin in the murine burn wound model of infection.APMIS. 2014 Feb;122(2):115-27. doi: 10.1111/apm.12101. Epub 2013 Jun 12. APMIS. 2014. PMID: 23758581
-
Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia. A new prophylactic principle.Behring Inst Mitt. 1997 Feb;(98):269-73. Behring Inst Mitt. 1997. PMID: 9382750 Review.
-
Vaccination against respiratory Pseudomonas aeruginosa infection.Hum Vaccin Immunother. 2015;11(1):14-20. doi: 10.4161/hv.34296. Epub 2014 Nov 1. Hum Vaccin Immunother. 2015. PMID: 25483510 Free PMC article. Review.
Cited by
-
Proteomic approach to identify host cell attachment proteins provides protective Pseudomonas aeruginosa vaccine antigen FtsZ.NPJ Vaccines. 2024 Oct 28;9(1):204. doi: 10.1038/s41541-024-00994-x. NPJ Vaccines. 2024. PMID: 39468053 Free PMC article.
-
Exploiting immunopotential PAPI-1 encoded type IVb major pilin targeting Pseudomonas aeruginosa.Heliyon. 2024 Aug 24;10(17):e36859. doi: 10.1016/j.heliyon.2024.e36859. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39281519 Free PMC article.
-
Defining the Mechanistic Correlates of Protection Conferred by Whole-Cell Vaccination against Pseudomonas aeruginosa Acute Murine Pneumonia.Infect Immun. 2021 Jan 19;89(2):e00451-20. doi: 10.1128/IAI.00451-20. Print 2021 Jan 19. Infect Immun. 2021. PMID: 33199354 Free PMC article.
-
Immunological considerations in the development of Pseudomonas aeruginosa vaccines.Hum Vaccin Immunother. 2020;16(2):412-418. doi: 10.1080/21645515.2019.1650999. Epub 2019 Sep 5. Hum Vaccin Immunother. 2020. PMID: 31368828 Free PMC article. Review.
-
Metallacarborane Derivatives Effective against Pseudomonas aeruginosa and Yersinia enterocolitica.Int J Mol Sci. 2021 Jun 23;22(13):6762. doi: 10.3390/ijms22136762. Int J Mol Sci. 2021. PMID: 34201818 Free PMC article.
References
-
- Douglas M. W., Mulholland K., Denyer V., Gottlieb T. Multi-drug resistant Pseudomonas aeruginosa outbreak in a burns unit—an infection control study. Burns : Journal of the International Society for Burn Injuries. 2001;27(2):131–135. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

