Regulation of Leukocyte Recruitment to the Spleen and Peritoneal Cavity during Pristane-Induced Inflammation
- PMID: 29201923
- PMCID: PMC5671734
- DOI: 10.1155/2017/9891348
Regulation of Leukocyte Recruitment to the Spleen and Peritoneal Cavity during Pristane-Induced Inflammation
Abstract
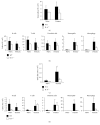
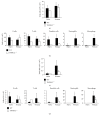
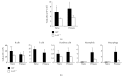
Chronic inflammation is associated with an increased number of leukocytes in the spleen, which are then redirected to the site of inflammation. However, it remains unknown how leukocyte recruitment is regulated. Herein, chronic inflammation was induced by intraperitoneal injection of pristane into mice. Leukocytes in the spleen or in the peritoneal cavity were quantified by flow cytometry. We found that the loss of IL-6 decreased macrophage recruitment to the spleen and the peritoneal cavity during pristane-induced inflammation. The loss of TNFα delayed the recruitment of neutrophils and macrophages to the spleen and inhibited the recruitment of neutrophils, macrophages, B cells, and T cells. The recruitment of neutrophils and macrophages into the spleen or peritoneal cavity was largely inhibited in the absence of LTα. The loss of TNFα receptor 1/2 resulted in reduced recruitment of neutrophils, macrophages, and dendritic cells into the spleen, but only neutrophil recruitment was inhibited in the peritoneal cavity. Similarly, a lack of B cells significantly impeded the recruitment of neutrophils, macrophages, and dendritic cells to the spleen. However, only macrophage recruitment was inhibited in the absence of T cells in the spleen. These data provide insight into the development of chronic inflammation induced by noninfectious substances.
Figures




Similar articles
-
Pristane-induced granulocyte recruitment promotes phenotypic conversion of macrophages and protects against diffuse pulmonary hemorrhage in Mac-1 deficiency.J Immunol. 2014 Nov 15;193(10):5129-39. doi: 10.4049/jimmunol.1401051. Epub 2014 Oct 3. J Immunol. 2014. PMID: 25281714
-
Endothelin-1 induces neutrophil recruitment in adaptive inflammation via TNFα and CXCL1/CXCR2 in mice.Can J Physiol Pharmacol. 2012 Feb;90(2):187-99. doi: 10.1139/y11-116. Epub 2012 Feb 9. Can J Physiol Pharmacol. 2012. PMID: 22320712
-
Neutrophil and macrophage responses to inflammation in the peritoneal cavity of rainbow trout Oncorhynchus mykiss. A light and electron microscopic cytochemical study.Dis Aquat Organ. 1998 Sep 11;34(1):27-37. doi: 10.3354/dao034027. Dis Aquat Organ. 1998. PMID: 9867437
-
The local inflammatory responses to infection of the peritoneal cavity in humans: their regulation by cytokines, macrophages, and other leukocytes.Mediators Inflamm. 2012;2012:976241. doi: 10.1155/2012/976241. Epub 2012 Feb 26. Mediators Inflamm. 2012. PMID: 22481867 Free PMC article. Review.
-
From leukocyte recruitment to resolution of inflammation: the cardinal role of integrins.J Leukoc Biol. 2017 Sep;102(3):677-683. doi: 10.1189/jlb.3MR0117-024R. Epub 2017 Mar 14. J Leukoc Biol. 2017. PMID: 28292945 Free PMC article. Review.
Cited by
-
Identification and characterization of miRNAs in spleens of sheep subjected to repetitive vaccination.Sci Rep. 2023 Apr 17;13(1):6239. doi: 10.1038/s41598-023-32603-7. Sci Rep. 2023. PMID: 37069162 Free PMC article.
-
The spleen size in patients undergoing hemodialysis.J Bras Nefrol. 2021 Jan-Mar;43(1):61-67. doi: 10.1590/2175-8239-JBN-2020-0116. J Bras Nefrol. 2021. PMID: 33079128 Free PMC article.
-
IOX1 impedes host inflammation in imiquimod-triggered psoriasis.Heliyon. 2021 Nov 19;7(11):e08433. doi: 10.1016/j.heliyon.2021.e08433. eCollection 2021 Nov. Heliyon. 2021. PMID: 34877426 Free PMC article.
-
Retinoic Acid Exerts Disease Stage-Dependent Effects on Pristane-Induced Lupus.Front Immunol. 2020 Mar 20;11:408. doi: 10.3389/fimmu.2020.00408. eCollection 2020. Front Immunol. 2020. PMID: 32265909 Free PMC article.
-
Central obesity and fat-free mass are associated with a larger spleen volume in the general population.Ups J Med Sci. 2024 May 29;129. doi: 10.48101/ujms.v129.10465. eCollection 2024. Ups J Med Sci. 2024. PMID: 38863728 Free PMC article.
References
-
- Potter M., Wax J. S. Genetics of susceptibility to pristane-induced plasmacytomas in BALB/cAn: reduced susceptibility in BALB/cJ with a brief description of pristane-induced arthritis. Journal of Immunology. 1981;127:1591–1595. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

