Cell-free Fluorescent Intra-Golgi Retrograde Vesicle Trafficking Assay
- PMID: 29201946
- PMCID: PMC5706631
- DOI: 10.21769/BioProtoc.2616
Cell-free Fluorescent Intra-Golgi Retrograde Vesicle Trafficking Assay
Abstract
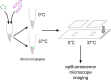
Intra-Golgi retrograde vesicle transport is used to traffic and sort resident Golgi enzymes to their appropriate cisternal locations. An assay was established to investigate the molecular details of vesicle targeting in a cell-free system. Stable cell lines were generated in which the trans-Golgi enzyme galactosyltransferase (GalT) was tagged with either CFP or YFP. Given that GalT is recycled to the cisterna where it is located at steady state, GalT-containing vesicles target GalT-containing cisternal membranes. Golgi membranes were therefore isolated from GalT-CFP expressing cells, while vesicles were prepared from GalT-YFP expressing ones. Incubating CFP-labelled Golgi with YFP-labelled vesicles in the presence of cytosol and an energy regeneration mixture at 37 °C produced a significant increase in CFP-YFP co-localization upon fluorescent imaging of the mixture compared to incubation on ice. The assay was validated to require energy, proteins and physiologically important trafficking components such as Rab GTPases and the conserved oligomeric Golgi tethering complex. This assay is useful for the investigation of both physiological and pathological changes that affect the Golgi trafficking machinery, in particular, vesicle tethering.
Keywords: Conserved oligomeric Golgi complex; Fluorescent imaging; Galactosyltransferase; Golgi apparatus; Vesicle tethering; Vesicle trafficking.
Conflict of interest statement
The authors declare that there’s no conflicts of interest.
Figures



Similar articles
-
Dissecting functions of the conserved oligomeric Golgi tethering complex using a cell-free assay.Traffic. 2014 Jan;15(1):12-21. doi: 10.1111/tra.12128. Epub 2013 Oct 31. Traffic. 2014. PMID: 24102787 Free PMC article.
-
The Golgi puppet master: COG complex at center stage of membrane trafficking interactions.Histochem Cell Biol. 2013 Sep;140(3):271-83. doi: 10.1007/s00418-013-1117-6. Epub 2013 Jul 10. Histochem Cell Biol. 2013. PMID: 23839779 Free PMC article. Review.
-
Electron tomography reveals Rab6 is essential to the trafficking of trans-Golgi clathrin and COPI-coated vesicles and the maintenance of Golgi cisternal number.Traffic. 2012 May;13(5):727-44. doi: 10.1111/j.1600-0854.2012.01343.x. Epub 2012 Mar 14. Traffic. 2012. PMID: 22335553 Free PMC article.
-
Bridging the Gap between Glycosylation and Vesicle Traffic.Front Cell Dev Biol. 2016 Mar 8;4:15. doi: 10.3389/fcell.2016.00015. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27014691 Free PMC article. Review.
-
Transition of galactosyltransferase 1 from trans-Golgi cisterna to the trans-Golgi network is signal mediated.Mol Biol Cell. 2006 Dec;17(12):5153-62. doi: 10.1091/mbc.e06-08-0665. Epub 2006 Oct 4. Mol Biol Cell. 2006. PMID: 17021253 Free PMC article.
References
-
- Balch W. E., Dunphy W. G., Braell W. A. and Rothman J. E.(1984). Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell 39(2Pt 1): 405-416. - PubMed
-
- Cottam N. P.(2012). A cell-free vesicle tethering assay. PhD thesis, University of York.
-
- Cottam N. P. and Ungar D.(2012). Retrograde vesicle transport in the Golgi. Protoplasma 249(4): 943-955. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

