Experimental data of co-crystals of Etravirine and L-tartaric acid
- PMID: 29201980
- PMCID: PMC5699872
- DOI: 10.1016/j.dib.2017.11.019
Experimental data of co-crystals of Etravirine and L-tartaric acid
Abstract
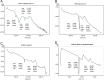
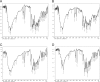
Etravirine is a drug used alongside other medication in the treatment of HIV and is a non-nucleoside reverse transcriptase inhibitor. It is a BCS class IV drug, having low solubility and high permeability (Drugbank, https://www.drugbank.ca/drugs/DB06414) [1]. As a result, large doses of the drug are required for treatment. Two pills have to be taken twice a day, making it a "pill burden" (Intelence, http://www.intelence.com/hcp/dosing/administration-options) [2]. Therefore, attempts of co-crystallizing Etravirine are attractive as the solubility of the drug tends to increase in this solid form (Schultheiss and Newman, 2009) [3]. In this study Etravirine co-crystals were synthesized in the molar ratios 1:1, 1:2 and 2:1 with L-tartaric acid as the co-former. Both slow evaporation and physical mixture was performed to mix the components. DSC values of final products are presented as well as FTIR spectra to observe the altered intermolecular interactions. A chemical stability test was performed after seven days using area under curve data from an HPLC instrument.
Keywords: Co-crystals; DSC instrument; Etravirine; FTIR instrument; HPLC.
Figures

References
-
- Drugbank, 〈https://www.drugbank.ca/drugs/DB06414〉, (Accessed 24 July 2017).
-
- Intelence, 〈http://www.intelence.com/hcp/dosing/administration-options〉, (Accessed 24 July 2017).
-
- Aithal K., Pai A., Pai G., Muddukrishna B.S. Preparation, solid state characterization of Etravirine co-crystals with improved solubility. Lat. Am. J. Pharm. 2017;36(5):972–979.
-
- Toronto Research Chemicals, 〈https://www.trc-canada.com/product-detail/?CatNum=E937000〉, (Accessed 24 July 2017).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

