Reversible Self-Assembled Monolayers (rSAMs): Adaptable Surfaces for Enhanced Multivalent Interactions and Ultrasensitive Virus Detection
- PMID: 29202022
- PMCID: PMC5704293
- DOI: 10.1021/acscentsci.7b00412
Reversible Self-Assembled Monolayers (rSAMs): Adaptable Surfaces for Enhanced Multivalent Interactions and Ultrasensitive Virus Detection
Abstract
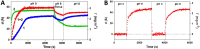
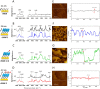
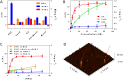
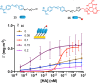
We report on the design of pH-switchable monolayers allowing a reversible and ordered introduction of affinity reagents on sensor surfaces. The principal layer building blocks consist of α-(4-amidinophenoxy)alkanes decorated at the ω-position with affinity ligands. These spontaneously self-assemble on top of carboxylic acid terminated SAMs to form reversible homo or mixed monolayers (rSAMs) that are tunable with respect to the nature of the head group, layer order and stability while featuring pH responsiveness and the dynamic nature of noncovalent build assemblies. We show that this results in a range of unique biosensor features. As a first example a sialic acid rSAM featuring strong lectin affinity is here used to sense hemagglutinin and influenza virus (H5N1) at the pM and fM level by in situ ellipsometry in a fully reversible fashion. We believe that the rSAM concept will find widespread use in surface chemistry and overall for boosting sensitivity in affinity biosensors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Spevak W.; Nagy J. O.; Charych D. H. Molecular assemblies of functionalized polydiacetylenes. Adv. Mater. 1995, 7, 85–89. 10.1002/adma.19950070120. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
