Through the Eustachian Tube and Beyond: A New Miniature Robotic Endoscope to See Into The Middle Ear
- PMID: 29202035
- PMCID: PMC5708577
- DOI: 10.1109/LRA.2017.2668468
Through the Eustachian Tube and Beyond: A New Miniature Robotic Endoscope to See Into The Middle Ear
Abstract
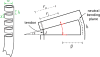
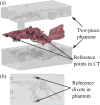
This paper presents a novel miniature robotic endoscope that is small enough to pass through the Eustachian tube and provide visualization of the middle ear (ME). The device features a miniature bending tip previously conceived of as a small-scale robotic wrist that has been adapted to carry and aim a small chip-tip camera and fiber optic light sources. The motivation for trans-Eustachian tube ME inspection is to provide a natural-orifice-based route to the ME that does not require cutting or lifting the eardrum, as is currently required. In this paper, we first perform an analysis of the ME anatomy and use a computational design optimization platform to derive the kinematic requirements for endoscopic inspection of the ME through the Eustachian tube. Based on these requirements, we fabricate the proposed device and use it to demonstrate the feasibility of ME inspection in an anthropomorphic model, i.e. a 3D-printed ME phantom generated from patient image data. We show that our prototype provides > 74% visibility coverage of the sinus tympani, a region of the ME crucial for diagnosis, compared to an average of only 6.9% using a straight, non-articulated endoscope through the Eustachian Tube.
Keywords: Medical Robots and Systems; Steerable Catheters/Needles; Surgical Robotics.
Figures








References
-
- Burgner-Kahrs J, Rucker DC, Choset H. Continuum robots for medical applications: A survey. IEEE Transactions on Robotics. 2015;31(6):1261–1280.
-
- Zhang D, Bennett ML, Labadie RF, Noble JH. Simulation of trans-nasal endoscopy of the middle ear for visualization of cholesteatoma. 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI) 2015 Apr;:1415–1418.
-
- Wallis S, Atkinson H, Coatesworth AP. Chronic otitis media. Postgraduate Medicine. 2015;127(4):391–395. - PubMed
-
- Browning GG, Gatehouse S. The prevalence of middle ear disease in the adult british population. Clinical Otolaryngology & Allied Sciences. 1992;17(4):317–321. - PubMed
-
- Hannula S, Bloigu R, Majamaa K, Sorri M, Mki-Torkko E. Ear diseases and other risk factors for hearing impairment among adults: an epidemiological study. International Journal of Audiology. 2012;51(11):833–840. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
