Global Transcriptome Analysis of Aedes aegypti Mosquitoes in Response to Zika Virus Infection
- PMID: 29202041
- PMCID: PMC5700376
- DOI: 10.1128/mSphere.00456-17
Global Transcriptome Analysis of Aedes aegypti Mosquitoes in Response to Zika Virus Infection
Abstract
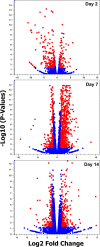
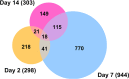
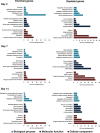
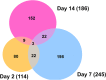
Zika virus (ZIKV) of the Flaviviridae family is a recently emerged mosquito-borne virus that has been implicated in the surge of the number of microcephaly instances in South America. The recent rapid spread of the virus led to its declaration as a global health emergency by the World Health Organization. The virus is transmitted mainly by the mosquito Aedes aegypti, which is also the vector of dengue virus; however, little is known about the interactions of the virus with the mosquito vector. In this study, we investigated the transcriptome profiles of whole A. aegypti mosquitoes in response to ZIKV infection at 2, 7, and 14 days postinfection using transcriptome sequencing. Results showed changes in the abundance of a large number of transcripts at each time point following infection, with 18 transcripts commonly changed among the three time points. Gene ontology analysis revealed that most of the altered genes are involved in metabolic processes, cellular processes, and proteolysis. In addition, 486 long intergenic noncoding RNAs that were altered upon ZIKV infection were identified. Further, we found changes of a number of potential mRNA target genes correlating with those of altered host microRNAs. The outcomes provide a basic understanding of A. aegypti responses to ZIKV and help to determine host factors involved in replication or mosquito host antiviral response against the virus. IMPORTANCE Vector-borne viruses pose great risks to human health. Zika virus has recently emerged as a global threat, rapidly expanding its distribution. Understanding the interactions of the virus with mosquito vectors at the molecular level is vital for devising new approaches in inhibiting virus transmission. In this study, we embarked on analyzing the transcriptional response of Aedes aegypti mosquitoes to Zika virus infection. Results showed large changes in both coding and long noncoding RNAs. Analysis of these genes showed similarities with other flaviviruses, including dengue virus, which is transmitted by the same mosquito vector. The outcomes provide a global picture of changes in the mosquito vector in response to Zika virus infection.
Keywords: Aedes aegypti; RNA-Seq; Zika virus; behavior; long noncoding RNA; microRNA; odorant binding protein; transcriptome.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

