Dihydrosanguinarine Enhances Glucose Uptake in Mouse 3T3-L1 Cells
- PMID: 29202114
- PMCID: PMC5705173
- DOI: 10.1021/acsomega.7b01134
Dihydrosanguinarine Enhances Glucose Uptake in Mouse 3T3-L1 Cells
Abstract
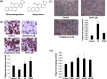
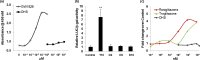
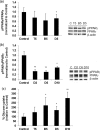
Recently, more studies have aimed at identifying selective peroxisome proliferator-activated receptor gamma (PPARγ) modulators that transactivate the expression of PPARγ-dependent genes as partial agonists to improve diabetic symptoms with fewer side effects compared to classic PPARγ agonists such as thiazolidinediones. We found that dihydrosanguinarine (DHS) treatment induced preadipocyte differentiation and lipid droplet accumulation in 3T3-L1 cells, but this effect is weaker than that elicited by the full PPARγ agonist troglitazone. Furthermore, this effect was reduced by the addition of a PPARγ antagonist, indicating the involvement of PPARγ signaling. Our results suggest that the stimulatory effects of DHS on adipocyte differentiation and insulin sensitivity are mediated by suppressing adenosine monophosphate-activated protein kinase (AMPK) alpha, upregulating the expression of PPARγ and its target genes (particularly Glut-4 and adiponectin) and reducing PPARγ phosphorylation. DHS significantly enhanced the glucose uptake in 3T3-L1 adipocytes without observable cytotoxicity at the effective concentration (5 μM) applied.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Magnolol enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells.Life Sci. 2009 Jun 19;84(25-26):908-14. doi: 10.1016/j.lfs.2009.04.001. Epub 2009 Apr 17. Life Sci. 2009. PMID: 19376135
-
Artepillin C, as a PPARγ ligand, enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells.Biochem Pharmacol. 2011 Apr 1;81(7):925-33. doi: 10.1016/j.bcp.2011.01.002. Epub 2011 Jan 8. Biochem Pharmacol. 2011. PMID: 21219874
-
A selective peroxisome proliferator-activated receptor-gamma (PPARgamma) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes.Mol Endocrinol. 2000 Sep;14(9):1425-33. doi: 10.1210/mend.14.9.0528. Mol Endocrinol. 2000. PMID: 10976920
-
WSF-7 Inhibits Obesity-Mediated PPARγ Phosphorylation and Improves Insulin Sensitivity in 3T3-L1 Adipocytes.Biol Pharm Bull. 2020;43(3):526-532. doi: 10.1248/bpb.b19-00986. Biol Pharm Bull. 2020. PMID: 32115511
-
Development of an In Vitro Screening Platform for the Identification of Partial PPARγ Agonists as a Source for Antidiabetic Lead Compounds.Molecules. 2018 Sep 22;23(10):2431. doi: 10.3390/molecules23102431. Molecules. 2018. PMID: 30248999 Free PMC article. Review.
Cited by
-
Neuropharmacological Effects of the Dichloromethane Extract from the Stems of Argemone ochroleuca Sweet (Papaveraceae) and Its Active Compound Dihydrosanguinarine.Pharmaceuticals (Basel). 2023 Aug 18;16(8):1175. doi: 10.3390/ph16081175. Pharmaceuticals (Basel). 2023. PMID: 37631090 Free PMC article.
-
Preliminary Phytochemical Screening, In Vitro Antidiabetic, Antioxidant Activities, and Toxicity of Leaf Extracts of Psychotria malayana Jack.Plants (Basel). 2021 Dec 7;10(12):2688. doi: 10.3390/plants10122688. Plants (Basel). 2021. PMID: 34961160 Free PMC article.
-
Quercetin-3-O-rutinoside from Moringa oleifera Downregulates Adipogenesis and Lipid Accumulation and Improves Glucose Uptake by Activation of AMPK/Glut-4 in 3T3-L1 Cells.Rev Bras Farmacogn. 2023;33(2):334-343. doi: 10.1007/s43450-022-00352-9. Epub 2023 Feb 13. Rev Bras Farmacogn. 2023. PMID: 36819090 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
