Nebulized Heparin Attenuates Pulmonary Coagulopathy and Inflammation through Alveolar Macrophages in a Rat Model of Acute Lung Injury
- PMID: 29202212
- PMCID: PMC6328369
- DOI: 10.1160/TH17-05-0347
Nebulized Heparin Attenuates Pulmonary Coagulopathy and Inflammation through Alveolar Macrophages in a Rat Model of Acute Lung Injury
Abstract
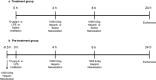
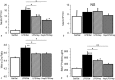
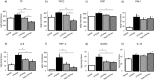
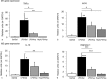
Objective Alveolar macrophages play a key role in the development and resolution of acute respiratory distress syndrome (ARDS), modulating the inflammatory response and the coagulation cascade in lungs. Anti-coagulants may be helpful in the treatment of ARDS. This study investigated the effects of nebulized heparin on the role of alveolar macrophages in limiting lung coagulation and inflammatory response in an animal model of acute lung injury (ALI). Methods Rats were randomized to four experimental groups. In three groups, ALI was induced by intratracheal instillation of lipopolysaccharide (LPS) and heparin was nebulized at constant oxygen flow: the LPS/Hep group received nebulized heparin 4 and 8 hours after injury; the Hep/LPS/Hep group received nebulized heparin 30 minutes before and 4 and 8 hours after LPS-induced injury; the LPS/Sal group received nebulized saline 4 and 8 hours after injury. The control group received only saline. Animals were exsanguinated 24 hours after LPS instillation. Lung tissue, bronchoalveolar lavage fluid (BALF) and alveolar macrophages isolated from BALF were analysed. Results LPS increased protein concentration, oedema and neutrophils in BALF as well as procoagulant and proinflammatory mediators in lung tissue and alveolar macrophages. In lung tissue, nebulized heparin attenuated ALI through decreasing procoagulant (tissue factor, thrombin-anti-thrombin complexes, fibrin degradation products) and proinflammatory (interleukin 6, tumour necrosis factor alpha) pathways. In alveolar macrophages, nebulized heparin reduced expression of procoagulant genes and the effectors of transforming growth factor beta (Smad 2, Smad 3) and nuclear factor kappa B (p-selectin, CCL-2). Pre-treatment resulted in more pronounced attenuation. Conclusion Nebulized heparin reduced pulmonary coagulopathy and inflammation without producing systemic bleeding, partly by modulating alveolar macrophages.
Schattauer GmbH Stuttgart.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.The work was performed in Institut d'Investigació i Innovació Parc Taulí (I3PT), Sabadell, Spain.
Figures

Similar articles
-
[The effects of three dosages of nebulized unfractionated heparin on alveolar coagulation and tissue inflammation injury in endotoxin-induced acute lung injury rat model].Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2012 Oct;24(10):612-5. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2012. PMID: 23040779 Chinese.
-
Myeloid but not epithelial tissue factor exerts protective anti-inflammatory effects in acid aspiration-induced acute lung injury.J Thromb Haemost. 2017 Aug;15(8):1625-1639. doi: 10.1111/jth.13737. Epub 2017 Jun 20. J Thromb Haemost. 2017. PMID: 28509332 Free PMC article.
-
Anti-inflammatory effects of novel curcumin analogs in experimental acute lung injury.Respir Res. 2015 Mar 24;16(1):43. doi: 10.1186/s12931-015-0199-1. Respir Res. 2015. PMID: 25889862 Free PMC article.
-
Alveolar-Capillary Membrane-Related Pulmonary Cells as a Target in Endotoxin-Induced Acute Lung Injury.Int J Mol Sci. 2019 Feb 15;20(4):831. doi: 10.3390/ijms20040831. Int J Mol Sci. 2019. PMID: 30769918 Free PMC article. Review.
-
Nebulized anticoagulants in lung injury in critically ill patients-an updated systematic review of preclinical and clinical studies.Ann Transl Med. 2017 Nov;5(22):444. doi: 10.21037/atm.2017.08.23. Ann Transl Med. 2017. PMID: 29264361 Free PMC article. Review.
Cited by
-
Exploring the therapeutic role of early heparin administration in ARDS management: a MIMIC-IV database analysis.J Intensive Care. 2024 Feb 26;12(1):9. doi: 10.1186/s40560-024-00723-5. J Intensive Care. 2024. PMID: 38409068 Free PMC article.
-
Cell therapy in acute respiratory distress syndrome.J Thorac Dis. 2018 Sep;10(9):5607-5620. doi: 10.21037/jtd.2018.08.28. J Thorac Dis. 2018. PMID: 30416812 Free PMC article. Review.
-
Future Trends in Nebulized Therapies for Pulmonary Disease.J Pers Med. 2020 May 10;10(2):37. doi: 10.3390/jpm10020037. J Pers Med. 2020. PMID: 32397615 Free PMC article. Review.
-
Priming Mesenchymal Stem Cells with Lipopolysaccharide Boosts the Immunomodulatory and Regenerative Activity of Secreted Extracellular Vesicles.Pharmaceutics. 2024 Oct 10;16(10):1316. doi: 10.3390/pharmaceutics16101316. Pharmaceutics. 2024. PMID: 39458645 Free PMC article.
-
Anticoagulant Treatment in Severe ARDS COVID-19 Patients.J Clin Med. 2022 May 10;11(10):2695. doi: 10.3390/jcm11102695. J Clin Med. 2022. PMID: 35628822 Free PMC article. Review.
References
-
- Bellani G, Laffey J G, Pham T et al.Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(08):788–800. - PubMed
-
- Rubenfeld G D, Caldwell E, Peabody E et al.Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. - PubMed
-
- Ranieri V M, Rubenfeld G D, Thompson B T et al.Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

