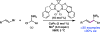Synthesis of Enantioenriched Allylic Silanes via Nickel-Catalyzed Reductive Cross-Coupling
- PMID: 29202243
- PMCID: PMC5851001
- DOI: 10.1021/jacs.7b11707
Synthesis of Enantioenriched Allylic Silanes via Nickel-Catalyzed Reductive Cross-Coupling
Abstract
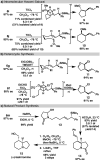
An asymmetric Ni-catalyzed reductive cross-coupling has been developed to prepare enantioenriched allylic silanes. This enantioselective reductive alkenylation proceeds under mild conditions and exhibits good functional group tolerance. The chiral allylic silanes prepared here undergo a variety of stereospecific transformations, including intramolecular Hosomi-Sakurai reactions, to set vicinal stereogenic centers with excellent transfer of chirality.
Figures
References
-
- Auner N, Weis J, editors. Organosilicon Chemistry V: From Molecules to Materials. Wiley-VCH; Weinheim, Germany: 2004.
-
-
Reviews: Chan TH, Wang D. Chem. Rev. 1992;92:995.Masse CE, Panek JS. Chem. Rev. 1995;95:1293.Fleming I, Barbero A, Walter D. Chem. Rev. 1997;97:2063.Chabaud L, James P, Landais Y. Eur. J. Org. Chem. 2004;15:3173.
-
-
- Hosomi A, Sakurai H. Tetrahedron Lett. 1976;17:1295.
- Hosomi A, Endo M, Sakurai H. Chem. Lett. 1976;5:941.
- Kira M, Kobayashi M, Sakurai H. Tetrahedron Lett. 1987;28:4081.
- Kira M, Sato K, Sakaurai H. J. Am. Chem. Soc. 1990;112:257.
- Chemler SR, Roush WR. J. Org. Chem. 1998;63:3800. - PubMed
-
-
Seminal reports: Hayashi T, Konishi M, Ito H, Kumada M. J. Am. Chem. Soc. 1982;104:4962.Hayashi T, Konishi M, Kumada M. J. Am. Chem. Soc. 1982;104:4963.Hayashi T, Konishi M, Okamoto Y, Kabeta K, Kumada M. J. Org. Chem. 1986;51:3772.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources