Angiotensin-converting enzyme defines matrikine-regulated inflammation and fibrosis
- PMID: 29202450
- PMCID: PMC5752376
- DOI: 10.1172/jci.insight.91923
Angiotensin-converting enzyme defines matrikine-regulated inflammation and fibrosis
Abstract
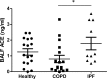
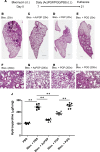
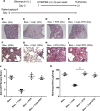
The neutrophil chemoattractant proline-glycine-proline (PGP) is generated from collagen by matrix metalloproteinase-8/9 (MMP-8/9) and prolyl endopeptidase (PE), and it is concomitantly degraded by extracellular leukotriene A4 hydrolase (LTA4H) to limit neutrophilia. Components of cigarette smoke can acetylate PGP, yielding a species (AcPGP) that is resistant to LTA4H-mediated degradation and can, thus, support a sustained neutrophilia. In this study, we sought to elucidate if an antiinflammatory system existed to degrade AcPGP that is analogous to the PGP-LTA4H axis. We demonstrate that AcPGP is degraded through a previously unidentified action of the enzyme angiotensin-converting enzyme (ACE). Pulmonary ACE is elevated during episodes of acute inflammation, as a consequence of enhanced vascular permeability, to ensure the efficient degradation of AcPGP. Conversely, we suggest that this pathway is aberrant in chronic obstructive pulmonary disease (COPD) enabling the accumulation of AcPGP. Consequently, we identify a potentially novel protective role for AcPGP in limiting pulmonary fibrosis and suggest the pathogenic function attributed to ACE in idiopathic pulmonary fibrosis (IPF) to be a consequence of overzealous AcPGP degradation. Thus, AcPGP seemingly has very divergent roles: it is pathogenic in its capacity to drive neutrophilic inflammation and matrix degradation in the context of COPD, but it is protective in its capacity to limit fibrosis in IPF.
Keywords: Fibrosis; Pulmonology.
Conflict of interest statement
Figures





References
-
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. - DOI - PubMed
-
- [No authors listed] American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL110950/HL/NHLBI NIH HHS/United States
- R01 HL114439/HL/NHLBI NIH HHS/United States
- R01 HL085324/HL/NHLBI NIH HHS/United States
- K08 HL092296/HL/NHLBI NIH HHS/United States
- R01 HL126596/HL/NHLBI NIH HHS/United States
- R35 HL135710/HL/NHLBI NIH HHS/United States
- T32 HL087738/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 HL127338/HL/NHLBI NIH HHS/United States
- R01 HL077783/HL/NHLBI NIH HHS/United States
- T32 GM008361/GM/NIGMS NIH HHS/United States
- P01 HL129941/HL/NHLBI NIH HHS/United States
- F30 HL136195/HL/NHLBI NIH HHS/United States
- T32 HL105346/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

