Increased Ca2+ signaling through CaV1.2 promotes bone formation and prevents estrogen deficiency-induced bone loss
- PMID: 29202453
- PMCID: PMC5752375
- DOI: 10.1172/jci.insight.95512
Increased Ca2+ signaling through CaV1.2 promotes bone formation and prevents estrogen deficiency-induced bone loss
Abstract
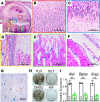
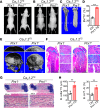
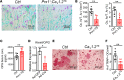
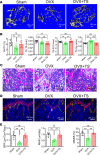
While the prevalence of osteoporosis is growing rapidly with population aging, therapeutic options remain limited. Here, we identify potentially novel roles for CaV1.2 L-type voltage-gated Ca2+ channels in osteogenesis and exploit a transgenic gain-of-function mutant CaV1.2 to stem bone loss in ovariectomized female mice. We show that endogenous CaV1.2 is expressed in developing bone within proliferating chondrocytes and osteoblasts. Using primary BM stromal cell (BMSC) cultures, we found that Ca2+ influx through CaV1.2 activates osteogenic transcriptional programs and promotes mineralization. We used Prx1-, Col2a1-, or Col1a1-Cre drivers to express an inactivation-deficient CaV1.2 mutant in chondrogenic and/or osteogenic precursors in vivo and found that the resulting increased Ca2+ influx markedly thickened bone not only by promoting osteogenesis, but also by inhibiting osteoclast activity through increased osteoprotegerin secretion from osteoblasts. Activating the CaV1.2 mutant in osteoblasts at the time of ovariectomy stemmed bone loss. Together, these data highlight roles for CaV1.2 in bone and demonstrate the potential dual anabolic and anticatabolic therapeutic actions of tissue-specific CaV1.2 activation in osteoblasts.
Keywords: Bone Biology; Bone development; Calcium channels; Osteoclast/osteoblast biology.
Conflict of interest statement
Figures

Similar articles
-
The CaV1.2 L-type calcium channel regulates bone homeostasis in the middle and inner ear.Bone. 2019 Aug;125:160-168. doi: 10.1016/j.bone.2019.05.024. Epub 2019 May 20. Bone. 2019. PMID: 31121355 Free PMC article.
-
Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomorphological study in ovariectomized rats.Bone. 2007 Feb;40(2):485-92. doi: 10.1016/j.bone.2006.09.004. Epub 2006 Oct 20. Bone. 2007. PMID: 17055793
-
Ablation of p38α MAPK Signaling in Osteoblast Lineage Cells Protects Mice From Bone Loss Induced by Estrogen Deficiency.Endocrinology. 2015 Dec;156(12):4377-87. doi: 10.1210/en.2015-1669. Epub 2015 Oct 6. Endocrinology. 2015. PMID: 26441240
-
The Role of FoxOs in Bone Health and Disease.Curr Top Dev Biol. 2018;127:149-163. doi: 10.1016/bs.ctdb.2017.10.004. Epub 2017 Dec 14. Curr Top Dev Biol. 2018. PMID: 29433736 Review.
-
Mechanisms involved in bone resorption regulated by vitamin D.J Steroid Biochem Mol Biol. 2018 Mar;177:70-76. doi: 10.1016/j.jsbmb.2017.11.005. Epub 2017 Nov 14. J Steroid Biochem Mol Biol. 2018. PMID: 29146302 Review.
Cited by
-
An L-type calcium channel blocker nimodipine exerts anti-fibrotic effects by attenuating TGF-β1 induced calcium response in an in vitro model of thyroid eye disease.Eye Vis (Lond). 2024 Sep 6;11(1):37. doi: 10.1186/s40662-024-00401-5. Eye Vis (Lond). 2024. PMID: 39237996 Free PMC article.
-
Voltage-Gated Calcium Channels in Nonexcitable Tissues.Annu Rev Physiol. 2021 Feb 10;83:183-203. doi: 10.1146/annurev-physiol-031620-091043. Epub 2020 Oct 26. Annu Rev Physiol. 2021. PMID: 33106102 Free PMC article. Review.
-
Convergence of Calcium Channel Regulation and Mechanotransduction in Skeletal Regenerative Biomaterial Design.Adv Healthc Mater. 2023 Oct;12(27):e2301081. doi: 10.1002/adhm.202301081. Epub 2023 Jul 16. Adv Healthc Mater. 2023. PMID: 37380172 Free PMC article. Review.
-
Comparative Proteomic and Metabolomic Analysis of Human Osteoblasts, Differentiated from Dental Pulp Stem Cells, Hinted Crucial Signaling Pathways Promoting Osteogenesis.Int J Mol Sci. 2021 Jul 24;22(15):7908. doi: 10.3390/ijms22157908. Int J Mol Sci. 2021. PMID: 34360674 Free PMC article.
-
STING suppresses bone cancer pain via immune and neuronal modulation.Nat Commun. 2021 Jul 27;12(1):4558. doi: 10.1038/s41467-021-24867-2. Nat Commun. 2021. PMID: 34315904 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

