A simple, clinically relevant therapeutic vaccine shows long-term protection in an aggressive, delayed-treatment B lymphoma model
- PMID: 29202455
- PMCID: PMC5752379
- DOI: 10.1172/jci.insight.92522
A simple, clinically relevant therapeutic vaccine shows long-term protection in an aggressive, delayed-treatment B lymphoma model
Abstract
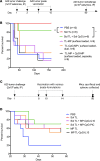
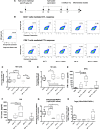
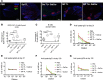
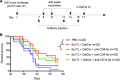
Despite initial remission after successful treatments, B lymphoma patients often encounter relapses and resistance causing high mortality. Thus, there is a need to develop therapies that prevent relapse by providing long-term protection and, ultimately, lead to functional cure. In this study, our goal was to develop a simple, clinically relevant, and easily translatable therapeutic vaccine that provides durable immune protection against aggressive B cell lymphoma and identify critical immune biomarkers that are predictive of long-term survival. In a delayed-treatment, aggressive, murine model of A20 B lymphoma that mimics human diffuse large B cell lymphoma, we show that therapeutic A20 lysate vaccine adjuvanted with an NKT cell agonist, α-galactosylceramide (α-GalCer), provides long-term immune protection against lethal tumor challenges and the antitumor immunity is primarily CD8 T cell dependent. Using experimental and computational methods, we demonstrate that the initial strength of germinal center reaction and the magnitude of class-switching into a Th1 type humoral response are the best predictors for the long-term immunity of B lymphoma lysate vaccine. Our results not only provide fundamentally insights for successful immunotherapy and long-term protection against B lymphomas, but also present a simple, therapeutic vaccine that can be translated easily due to the facile and inexpensive method of preparation.
Keywords: Cancer immunotherapy; Immunology; Lymphomas; Vaccines.
Conflict of interest statement
Figures


Similar articles
-
NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma.Blood. 2012 Oct 11;120(15):3019-29. doi: 10.1182/blood-2012-04-426643. Epub 2012 Aug 28. Blood. 2012. PMID: 22932803 Free PMC article.
-
Dendritic cells combined with tumor cells and α-galactosylceramide induce a potent, therapeutic and NK-cell dependent antitumor immunity in B cell lymphoma.J Transl Med. 2017 May 26;15(1):115. doi: 10.1186/s12967-017-1219-3. J Transl Med. 2017. PMID: 28549432 Free PMC article.
-
An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity.Blood. 2007 Sep 15;110(6):2013-9. doi: 10.1182/blood-2006-12-061309. Epub 2007 Jun 20. Blood. 2007. PMID: 17581919 Free PMC article.
-
Idiotypic vaccination for B-cell malignancies as a model for therapeutic cancer vaccines: from prototype protein to second generation vaccines.Haematologica. 2002 Sep;87(9):989-1001. Haematologica. 2002. PMID: 12217812 Review.
-
Optimization of natural killer T cell-mediated immunotherapy in cancer using cell-based and nanovector vaccines.Cancer Res. 2014 Mar 15;74(6):1632-8. doi: 10.1158/0008-5472.CAN-13-3504. Epub 2014 Mar 5. Cancer Res. 2014. PMID: 24599135 Review.
Cited by
-
Modulation of the gut microbiota engages antigen cross-presentation to enhance antitumor effects of CAR T cell immunotherapy.Mol Ther. 2023 Mar 1;31(3):686-700. doi: 10.1016/j.ymthe.2023.01.012. Epub 2023 Jan 14. Mol Ther. 2023. PMID: 36641624 Free PMC article.
-
Therapeutic Vaccines for Hematological Cancers: A Scoping Review of This Immunotherapeutic Approach as Alternative to the Treatment of These Malignancies.Vaccines (Basel). 2025 Jan 23;13(2):114. doi: 10.3390/vaccines13020114. Vaccines (Basel). 2025. PMID: 40006660 Free PMC article. Review.
-
TRAF6-IRF5 kinetics, TRIF, and biophysical factors drive synergistic innate responses to particle-mediated MPLA-CpG co-presentation.Sci Adv. 2021 Jan 13;7(3):eabd4235. doi: 10.1126/sciadv.abd4235. Print 2021 Jan. Sci Adv. 2021. PMID: 33523878 Free PMC article.
-
Invariant NKT cell-augmented GM-CSF-secreting tumor vaccine is effective in advanced prostate cancer model.Cancer Immunol Immunother. 2022 Dec;71(12):2943-2955. doi: 10.1007/s00262-022-03210-8. Epub 2022 May 6. Cancer Immunol Immunother. 2022. PMID: 35523889 Free PMC article.
-
Therapeutic vaccines for aggressive B-cell lymphoma.Leuk Lymphoma. 2020 Dec;61(13):3038-3051. doi: 10.1080/10428194.2020.1805113. Epub 2020 Aug 25. Leuk Lymphoma. 2020. PMID: 32840404 Free PMC article.
References
-
- Caspar CB, Levy S, Levy R. Idiotype vaccines for non-Hodgkin’s lymphoma induce polyclonal immune responses that cover mutated tumor idiotypes: comparison of different vaccine formulations. Blood. 1997;90(9):3699–3706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

