Endothelial chimerism and vascular sequestration protect pancreatic islet grafts from antibody-mediated rejection
- PMID: 29202467
- PMCID: PMC5749508
- DOI: 10.1172/JCI93542
Endothelial chimerism and vascular sequestration protect pancreatic islet grafts from antibody-mediated rejection
Abstract
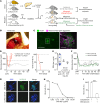
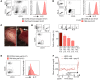
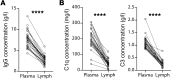
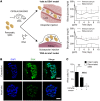
Humoral rejection is the most common cause of solid organ transplant failure. Here, we evaluated a cohort of 49 patients who were successfully grafted with allogenic islets and determined that the appearance of donor-specific anti-HLA antibodies (DSAs) did not accelerate the rate of islet graft attrition, suggesting resistance to humoral rejection. Murine DSAs bound to allogeneic targets expressed by islet cells and induced their destruction in vitro; however, passive transfer of the same DSAs did not affect islet graft survival in murine models. Live imaging revealed that DSAs were sequestrated in the circulation of the recipients and failed to reach the endocrine cells of grafted islets. We used murine heart transplantation models to confirm that endothelial cells were the only accessible targets for DSAs, which induced the development of typical microvascular lesions in allogeneic transplants. In contrast, the vasculature of DSA-exposed allogeneic islet grafts was devoid of lesions because sprouting of recipient capillaries reestablished blood flow in grafted islets. Thus, we conclude that endothelial chimerism combined with vascular sequestration of DSAs protects islet grafts from humoral rejection. The reduced immunoglobulin concentrations in the interstitial tissue, confirmed in patients, may have important implications for biotherapies such as vaccines and monoclonal antibodies.
Keywords: Immunoglobulins; Immunology; Islet cells; Transplantation.
Conflict of interest statement
Figures




References
-
- Kemp CB, Knight MJ, Scharp DW, Lacy PE, Ballinger WF. Transplantation of isolated pancreatic islets into the portal vein of diabetic rats. Nature. 1973;244(5416):447. - PubMed
-
- Scharp DW, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990;39(4):515–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

