Polycomb subunit BMI1 determines uterine progesterone responsiveness essential for normal embryo implantation
- PMID: 29202468
- PMCID: PMC5749512
- DOI: 10.1172/JCI92862
Polycomb subunit BMI1 determines uterine progesterone responsiveness essential for normal embryo implantation
Abstract
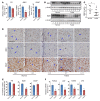
Natural and synthetic progestogens have been commonly used to prevent recurrent pregnancy loss in women with inadequate progesterone secretion or reduced progesterone sensitivity. However, the clinical efficacy of progesterone and its analogs for maintaining pregnancy is variable. Additionally, the underlying cause of impaired endometrial progesterone responsiveness during early pregnancy remains unknown. Here, we demonstrated that uterine-selective depletion of BMI1, a key component of the polycomb repressive complex-1 (PRC1), hampers uterine progesterone responsiveness and derails normal uterine receptivity, resulting in implantation failure in mice. We further uncovered genetic and biochemical evidence that BMI1 interacts with the progesterone receptor (PR) and the E3 ligase E6AP in a polycomb complex-independent manner and regulates the PR ubiquitination that is essential for normal progesterone responsiveness. A close association of aberrantly low endometrial BMI1 expression with restrained PR responsiveness in women who had previously had a miscarriage indicated that the role of BMI1 in endometrial PR function is conserved in mice and in humans. In addition to uncovering a potential regulatory mechanism of BMI1 that ensures normal endometrial progesterone responsiveness during early pregnancy, our findings have the potential to help clarify the underlying causes of spontaneous pregnancy loss in women.
Keywords: Fertility; Mouse models; Reproductive Biology; Sex hormones.
Conflict of interest statement
Figures



Similar articles
-
P38α MAPK is a gatekeeper of uterine progesterone responsiveness at peri-implantation via Ube3c-mediated PGR degradation.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2206000119. doi: 10.1073/pnas.2206000119. Epub 2022 Aug 1. Proc Natl Acad Sci U S A. 2022. PMID: 35914132 Free PMC article.
-
Targeted Degradation of PRC1 Components, BMI1 and RING1B, via a Novel Protein Complex Degrader Strategy.Adv Sci (Weinh). 2023 Apr;10(10):e2205573. doi: 10.1002/advs.202205573. Epub 2023 Feb 3. Adv Sci (Weinh). 2023. PMID: 36737841 Free PMC article.
-
Role of nuclear progesterone receptor isoforms in uterine pathophysiology.Hum Reprod Update. 2015 Mar-Apr;21(2):155-73. doi: 10.1093/humupd/dmu056. Epub 2014 Nov 18. Hum Reprod Update. 2015. PMID: 25406186 Free PMC article. Review.
-
Structure and E3-ligase activity of the Ring-Ring complex of polycomb proteins Bmi1 and Ring1b.EMBO J. 2006 Jun 7;25(11):2465-74. doi: 10.1038/sj.emboj.7601144. Epub 2006 May 18. EMBO J. 2006. PMID: 16710298 Free PMC article.
-
90 YEARS OF PROGESTERONE: New insights into progesterone receptor signaling in the endometrium required for embryo implantation.J Mol Endocrinol. 2020 Jul;65(1):T1-T14. doi: 10.1530/JME-19-0212. J Mol Endocrinol. 2020. PMID: 31809260 Free PMC article. Review.
Cited by
-
METTL3 is essential for normal progesterone signaling during embryo implantation via m6A-mediated translation control of progesterone receptor.Proc Natl Acad Sci U S A. 2023 Jan 31;120(5):e2214684120. doi: 10.1073/pnas.2214684120. Epub 2023 Jan 24. Proc Natl Acad Sci U S A. 2023. PMID: 36693099 Free PMC article.
-
Molecular mechanism of aberrant decidualization in adenomyosis leading to reduced endometrial receptivity.Front Endocrinol (Lausanne). 2025 Jan 16;15:1435177. doi: 10.3389/fendo.2024.1435177. eCollection 2024. Front Endocrinol (Lausanne). 2025. PMID: 39886033 Free PMC article. Review.
-
Histone modifications in embryo implantation and placentation: insights from mouse models.Front Endocrinol (Lausanne). 2023 Aug 4;14:1229862. doi: 10.3389/fendo.2023.1229862. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37600694 Free PMC article. Review.
-
Alpha-tocopherol enhances spermatogonial stem cell proliferation and restores mouse spermatogenesis by up-regulating BMI1.Front Nutr. 2023 Apr 17;10:1141964. doi: 10.3389/fnut.2023.1141964. eCollection 2023. Front Nutr. 2023. PMID: 37139440 Free PMC article.
-
Epigenetic control of embryo-uterine crosstalk at peri-implantation.Cell Mol Life Sci. 2019 Dec;76(24):4813-4828. doi: 10.1007/s00018-019-03245-8. Epub 2019 Jul 27. Cell Mol Life Sci. 2019. PMID: 31352535 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

