H3K27me3 dynamics dictate evolving uterine states in pregnancy and parturition
- PMID: 29202469
- PMCID: PMC5749543
- DOI: 10.1172/JCI95937
H3K27me3 dynamics dictate evolving uterine states in pregnancy and parturition
Abstract
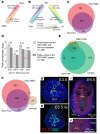
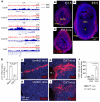
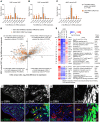
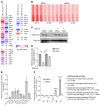
Uncovering the causes of pregnancy complications such as preterm labor requires greater insight into how the uterus remains in a noncontractile state until term and then surmounts this state to enter labor. Here, we show that dynamic generation and erasure of the repressive histone modification tri-methyl histone H3 lysine 27 (H3K27me3) in decidual stromal cells dictate both elements of pregnancy success in mice. In early gestation, H3K27me3-induced transcriptional silencing of select gene targets ensured uterine quiescence by preventing the decidua from expressing parturition-inducing hormone receptors, manifesting type 1 immunity, and most unexpectedly, generating myofibroblasts and associated wound-healing responses. In late gestation, genome-wide H3K27 demethylation allowed for target gene upregulation, decidual activation, and labor entry. Pharmacological inhibition of H3K27 demethylation in late gestation not only prevented term parturition, but also inhibited delivery while maintaining pup viability in a noninflammatory model of preterm parturition. Immunofluorescence analysis of human specimens suggested that similar regulatory events might occur in the human decidua. Together, these results reveal the centrality of regulated gene silencing in the uterine adaptation to pregnancy and suggest new areas in the study and treatment of pregnancy disorders.
Keywords: Epigenetics; Fibrosis; Obstetrics/gynecology; Reproductive Biology.
Conflict of interest statement
Figures

Similar articles
-
Gene silencing by EZH2 suppresses TGF-β activity within the decidua to avert pregnancy-adverse wound healing at the maternal-fetal interface.Cell Rep. 2022 Feb 1;38(5):110329. doi: 10.1016/j.celrep.2022.110329. Cell Rep. 2022. PMID: 35108527 Free PMC article.
-
KDM6B-dependent epigenetic programming of uterine fibroblasts in early pregnancy regulates parturition timing in mice.Cell. 2025 Mar 6;188(5):1265-1279.e18. doi: 10.1016/j.cell.2024.12.019. Epub 2025 Jan 21. Cell. 2025. PMID: 39842437
-
Genes upregulated in the amnion at labour are bivalently marked by activating and repressive histone modifications.Mol Hum Reprod. 2019 Apr 1;25(4):228-240. doi: 10.1093/molehr/gaz007. Mol Hum Reprod. 2019. PMID: 30753586
-
Topographical specialization of prostaglandin function in late pregnancy and at parturition in the baboon.Prostaglandins Leukot Essent Fatty Acids. 2004 Feb;70(2):199-206. doi: 10.1016/j.plefa.2003.04.008. Prostaglandins Leukot Essent Fatty Acids. 2004. PMID: 14683693 Review.
-
Epigenetic regulations through DNA methylation and hydroxymethylation: clues for early pregnancy in decidualization.Biomol Concepts. 2014 May;5(2):95-107. doi: 10.1515/bmc-2013-0036. Biomol Concepts. 2014. PMID: 25372745 Free PMC article. Review.
Cited by
-
Macrophage plasticity and function in cancer and pregnancy.Front Immunol. 2024 Jan 11;14:1333549. doi: 10.3389/fimmu.2023.1333549. eCollection 2023. Front Immunol. 2024. PMID: 38274812 Free PMC article. Review.
-
Proteogenomic and V(D)J Analysis of Human Decidual T Cells Highlights Unique Transcriptional Programming and Clonal Distribution.J Immunol. 2023 Jul 1;211(1):154-162. doi: 10.4049/jimmunol.2200061. J Immunol. 2023. PMID: 37195197 Free PMC article.
-
Epigenetic modifications working in the decidualization and endometrial receptivity.Cell Mol Life Sci. 2020 Jun;77(11):2091-2101. doi: 10.1007/s00018-019-03395-9. Epub 2019 Dec 7. Cell Mol Life Sci. 2020. PMID: 31813015 Free PMC article. Review.
-
Human Chorionic Gonadotropin modulates CXCL10 Expression through Histone Methylation in human decidua.Sci Rep. 2020 Apr 1;10(1):5785. doi: 10.1038/s41598-020-62593-9. Sci Rep. 2020. PMID: 32238853 Free PMC article.
-
Multi-omics-based mapping of decidualization resistance in patients with a history of severe preeclampsia.Nat Med. 2025 Feb;31(2):502-513. doi: 10.1038/s41591-024-03407-7. Epub 2025 Jan 7. Nat Med. 2025. PMID: 39775038 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

