Stromal Lkb1 deficiency leads to gastrointestinal tumorigenesis involving the IL-11-JAK/STAT3 pathway
- PMID: 29202476
- PMCID: PMC5749537
- DOI: 10.1172/JCI93597
Stromal Lkb1 deficiency leads to gastrointestinal tumorigenesis involving the IL-11-JAK/STAT3 pathway
Abstract
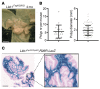
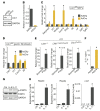
Germline mutations in the gene encoding tumor suppressor kinase LKB1 lead to gastrointestinal tumorigenesis in Peutz-Jeghers syndrome (PJS) patients and mouse models; however, the cell types and signaling pathways underlying tumor formation are unknown. Here, we demonstrated that mesenchymal progenitor- or stromal fibroblast-specific deletion of Lkb1 results in fully penetrant polyposis in mice. Lineage tracing and immunohistochemical analyses revealed clonal expansion of Lkb1-deficient myofibroblast-like cell foci in the tumor stroma. Loss of Lkb1 in stromal cells was associated with induction of an inflammatory program including IL-11 production and activation of the JAK/STAT3 pathway in tumor epithelia concomitant with proliferation. Importantly, treatment of LKB1-defcient mice with the JAK1/2 inhibitor ruxolitinib dramatically decreased polyposis. These data indicate that IL-11-mediated induction of JAK/STAT3 is critical in gastrointestinal tumorigenesis following Lkb1 mutations and suggest that targeting this pathway has therapeutic potential in Peutz-Jeghers syndrome.
Keywords: Gastric cancer; Gastroenterology; Mouse models; Oncology; Tumor suppressors.
Conflict of interest statement
Figures





Similar articles
-
LKB1 deficiency in T cells promotes the development of gastrointestinal polyposis.Science. 2018 Jul 27;361(6400):406-411. doi: 10.1126/science.aan3975. Science. 2018. PMID: 30049881
-
Tumor suppressor LKB1 inhibits activation of signal transducer and activator of transcription 3 (STAT3) by thyroid oncogenic tyrosine kinase rearranged in transformation (RET)/papillary thyroid carcinoma (PTC).Mol Endocrinol. 2007 Dec;21(12):3039-49. doi: 10.1210/me.2007-0269. Epub 2007 Aug 30. Mol Endocrinol. 2007. PMID: 17761947
-
Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation.Nature. 2002 Sep 12;419(6903):162-7. doi: 10.1038/nature01045. Nature. 2002. PMID: 12226664
-
LKB1 deficiency-induced metabolic reprogramming in tumorigenesis and non-neoplastic diseases.Mol Metab. 2021 Feb;44:101131. doi: 10.1016/j.molmet.2020.101131. Epub 2020 Dec 3. Mol Metab. 2021. PMID: 33278637 Free PMC article. Review.
-
Energy sensing and cancer: LKB1 function and lessons learnt from Peutz-Jeghers syndrome.Semin Cell Dev Biol. 2016 Apr;52:21-9. doi: 10.1016/j.semcdb.2016.02.015. Epub 2016 Feb 10. Semin Cell Dev Biol. 2016. PMID: 26877140 Review.
Cited by
-
Cancer Risk of Peutz-Jeghers Syndrome and Treatment Experience: A Chinese Medical Center.Clin Colon Rectal Surg. 2023 May 3;36(6):406-414. doi: 10.1055/s-0043-1767704. eCollection 2023 Nov. Clin Colon Rectal Surg. 2023. PMID: 37795464 Free PMC article. Review.
-
Embryonic Kidney Development, Stem Cells and the Origin of Wilms Tumor.Genes (Basel). 2021 Feb 23;12(2):318. doi: 10.3390/genes12020318. Genes (Basel). 2021. PMID: 33672414 Free PMC article. Review.
-
Antibody-drug conjugates plus Janus kinase inhibitors enable MHC-mismatched allogeneic hematopoietic stem cell transplantation.J Clin Invest. 2021 Dec 15;131(24):e145501. doi: 10.1172/JCI145501. J Clin Invest. 2021. PMID: 34730109 Free PMC article.
-
The mesenchymal context in inflammation, immunity and cancer.Nat Immunol. 2020 Sep;21(9):974-982. doi: 10.1038/s41590-020-0741-2. Epub 2020 Aug 3. Nat Immunol. 2020. PMID: 32747813 Review.
-
The Gastrointestinal Tumor Microenvironment: An Updated Biological and Clinical Perspective.J Oncol. 2019 Nov 22;2019:6240505. doi: 10.1155/2019/6240505. eCollection 2019. J Oncol. 2019. PMID: 31885581 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

