PMP22 antisense oligonucleotides reverse Charcot-Marie-Tooth disease type 1A features in rodent models
- PMID: 29202483
- PMCID: PMC5749515
- DOI: 10.1172/JCI96499
PMP22 antisense oligonucleotides reverse Charcot-Marie-Tooth disease type 1A features in rodent models
Abstract
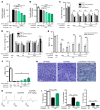
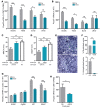
Charcot-Marie-Tooth disease type 1A (CMT1A) is caused by duplication of peripheral myelin protein 22 (PMP22) and is the most common hereditary peripheral neuropathy. CMT1A is characterized by demyelination and axonal loss, which underlie slowed motor nerve conduction velocity (MNCV) and reduced compound muscle action potentials (CMAP) in patients. There is currently no known treatment for this disease. Here, we show that antisense oligonucleotides (ASOs) effectively suppress PMP22 mRNA in affected nerves in 2 murine CMT1A models. Notably, initiation of ASO treatment after disease onset restored myelination, MNCV, and CMAP almost to levels seen in WT animals. In addition to disease-associated gene expression networks that were restored with ASO treatment, we also identified potential disease biomarkers through transcriptomic profiling. Furthermore, we demonstrated that reduction of PMP22 mRNA in skin biopsies from ASO-treated rats is a suitable biomarker for evaluating target engagement in response to ASO therapy. These results support the use of ASOs as a potential treatment for CMT1A and elucidate potential disease and target engagement biomarkers for use in future clinical trials.
Keywords: Drug therapy; Gene therapy; Monogenic diseases; Neuroscience; Therapeutics.
Conflict of interest statement
Figures

Comment in
-
Antisense oligonucleotides offer hope to patients with Charcot-Marie-Tooth disease type 1A.J Clin Invest. 2018 Jan 2;128(1):110-112. doi: 10.1172/JCI98617. Epub 2017 Dec 4. J Clin Invest. 2018. PMID: 29199996 Free PMC article.
-
Peripheral neuropathies: Antisense therapy for Charcot-Marie-Tooth disease?Nat Rev Neurol. 2018 Feb;14(2):64. doi: 10.1038/nrneurol.2017.182. Epub 2017 Dec 22. Nat Rev Neurol. 2018. PMID: 29269786 No abstract available.
References
-
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet. 1974;6(2):98–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

