Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial
- PMID: 29202655
- PMCID: PMC5880701
- DOI: 10.1176/appi.ajp.2017.17060647
Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial
Abstract
Objective: Pharmacotherapy to rapidly relieve suicidal ideation in depression may reduce suicide risk. Rapid reduction in suicidal thoughts after ketamine treatment has mostly been studied in patients with low levels of suicidal ideation. The authors tested the acute effect of adjunctive subanesthetic intravenous ketamine on clinically significant suicidal ideation in patients with major depressive disorder.
Method: In a randomized clinical trial, adults (N=80) with current major depressive disorder and a score ≥4 on the Scale for Suicidal Ideation (SSI), of whom 54% (N=43) were taking antidepressant medication, were randomly assigned to receive ketamine or midazolam infusion. The primary outcome measure was SSI score 24 hours after infusion (at day 1).
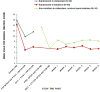
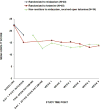
Results: The reduction in SSI score at day 1 was 4.96 points greater for the ketamine group compared with the midazolam group (95% CI=2.33, 7.59; Cohen's d=0.75). The proportion of responders (defined as having a reduction ≥50% in SSI score) at day 1 was 55% for the ketamine group and 30% for the midazolam group (odds ratio=2.85, 95% CI=1.14, 7.15; number needed to treat=4.0). Improvement in the Profile of Mood States depression subscale was greater at day 1 for the ketamine group compared with the midazolam group (estimate=7.65, 95% CI=1.36, 13.94), and this effect mediated 33.6% of ketamine's effect on SSI score. Side effects were short-lived, and clinical improvement was maintained for up to 6 weeks with additional optimized standard pharmacotherapy in an uncontrolled follow-up.
Conclusions: Adjunctive ketamine demonstrated a greater reduction in clinically significant suicidal ideation in depressed patients within 24 hours compared with midazolam, partially independently of antidepressant effect.
Trial registration: ClinicalTrials.gov NCT01700829.
Keywords: Clinical Trial; Depression; Ketamine; Suicidal Ideation.
Conflict of interest statement
Figures
Comment in
-
Ketamine: Quo Vadis?Am J Psychiatry. 2018 Apr 1;175(4):297-299. doi: 10.1176/appi.ajp.2018.18010014. Am J Psychiatry. 2018. PMID: 29606064 No abstract available.
References
-
- Fatal Injury Reports, National, Regional and State, 1981 – 2015. Centers for Disease Control and Prevention; [Accessed 05/24/2017]. URL: https://webappa.cdc.gov/sasweb/ncipc/mortrate.html.
-
- Jacobs DG, Baldessarini RJ, Conwell Y, Fawcett JA, Horton L, Meltzer H, Pfeffer CR, Simon RI. Practice Guideline for the Assessment and Treatment of Patients with Suicidal Behaviors. American Psychiatric Publishing, Inc.; 2003.
-
- Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer JP, Potkin S International Suicide Prevention Trial Study G. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) Arch Gen Psychiatry. 2003;60:82–91. - PubMed
-
- Prudic J, Sackeim HA. Electroconvulsive therapy and suicide risk. J Clin Psychiatry. 1999;60(Suppl 2):104–110. discussion 111–106. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

